This document describes several common tests used to identify different types of carbohydrates:
- Molisch test is a general test for all carbohydrates that involves using concentrated sulfuric acid and α-naphthol to form colored compounds.
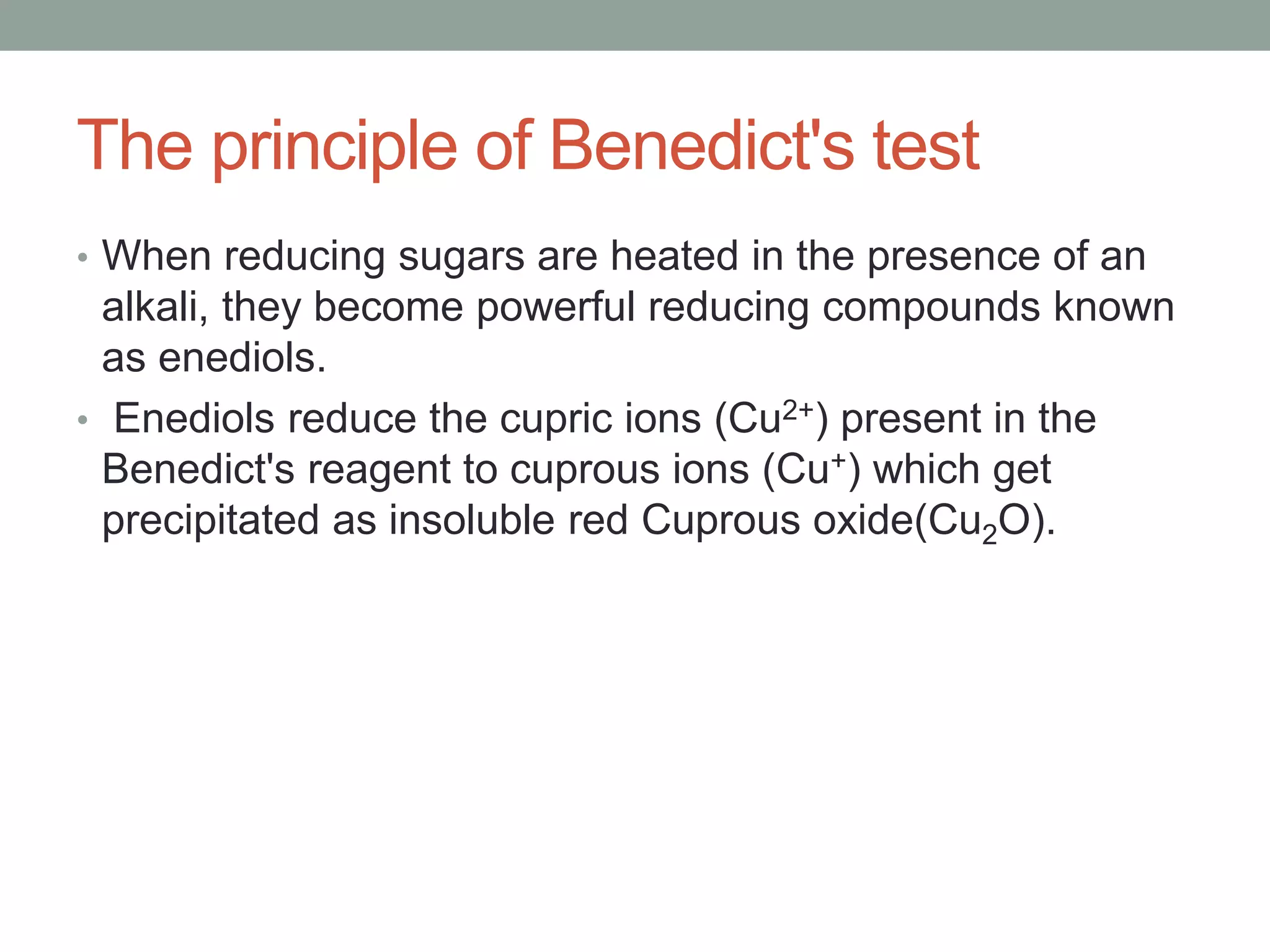
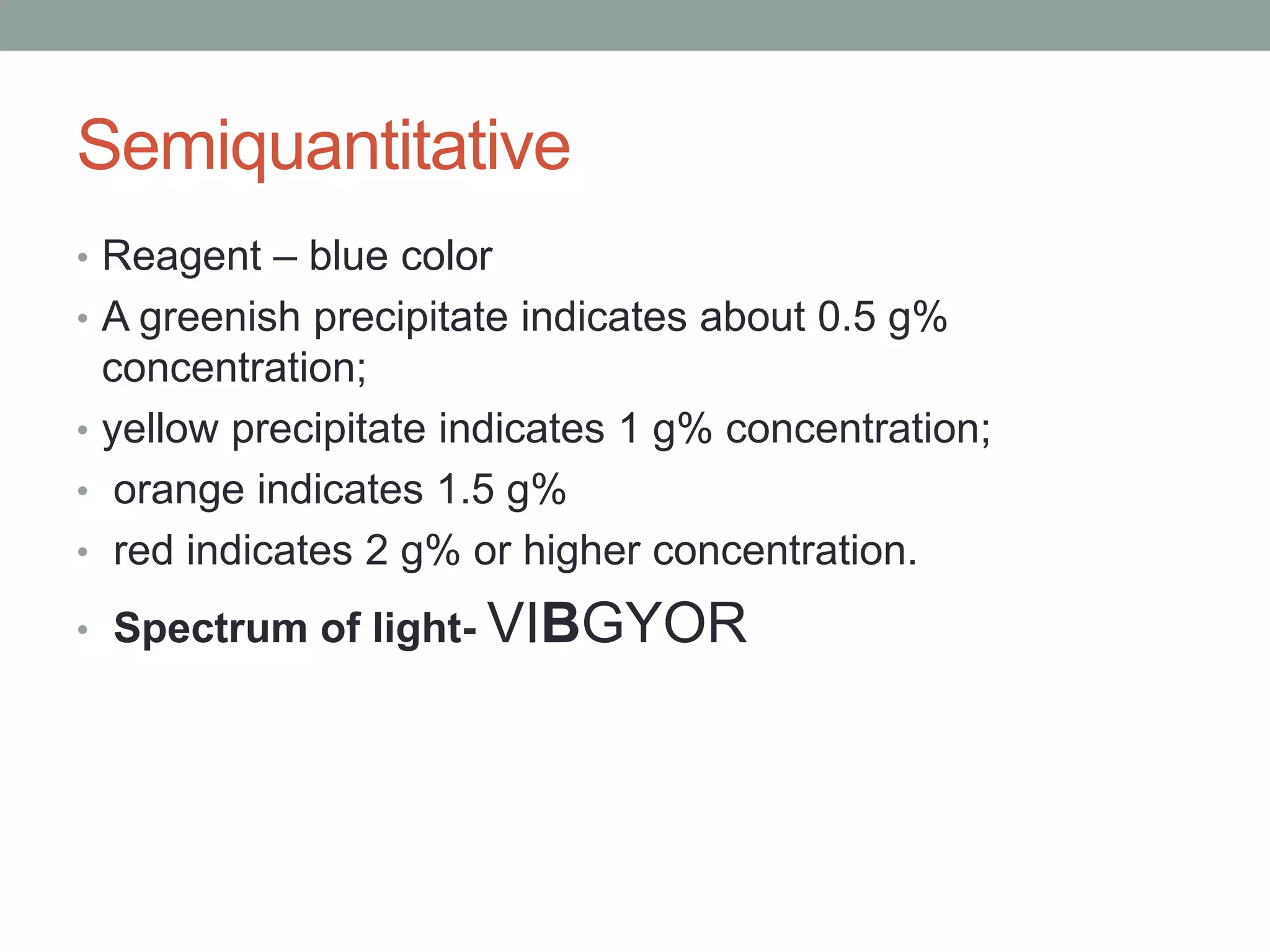
- Benedict's test uses copper sulfate and heat to detect reducing sugars, forming a red copper (II) oxide precipitate.
- Bial's test detects pentoses using orcinol and ferric ions to form a blue color.
- Osazone tests form characteristic crystal shapes when reducing sugars are heated with phenylhydrazine.
- Mucic acid test detects galactose by forming a white precipitate of mucic acid with nitric