The document discusses acid-base equilibria, including calculations for the pH of strong acids and strong bases, and how to find the pH of solutions resulting from mixing various acids and bases. It also covers the properties of salts derived from weak and strong acids/bases, buffer solutions, and polyprotic acids. Various calculations and examples illustrate the concepts of pH determination, buffer capacity, and the relationships between acidity constants.


![3
Find the pH of a 0.1 M HCl solution.
HCl is a strong acid that completely
dissociates in water, therefore we have
HCl H+ + Cl-
H2O D H+ + OH-
[H+]Solution = [H+]from HCl + [H+]from water
However, [H+]from water = 10-7 in absence of a
common ion, therefore it will be much less in
presence of HCl and can thus be neglected
as compared to 0.1 ( 0.1>>[H+]from water)
[H+]solution = [H+]HCl = 0.1M
pH = -log 0.1 = 1](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-3-320.jpg)

![5
Find the pH of a 10-10 M HCl solution.
HCl H+ + Cl-
H2O D H+ + OH-
[H+]Solution = [H+]from HCl + [H+]from water
pH = 7](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-5-320.jpg)
![6
Calculate the pH of the solution resulting from
mixing 50 mL of 0.1 M HCl with 50 mL of 0.2 M
NaOH.
When HCl is mixed with NaOH neutralization takes
place where they react in a 1:1 mole ratio.
Therefore, find mmol of each reagent to see if
there is an excess of either reagent
mmol HCl = 0.1 x 50 = 5 mmol
mmol NaOH = 0.2 x 50 = 10 mmol
mmol NaOH excess = 10 – 5 = 5 mmol
[OH-] = 5/100 = 0.05 M
pOH = 1.3 and pH = 14 – pOH = 14 – 1.3 = 12.7](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-6-320.jpg)
![7
Find the pH of a solution prepared by mixing 2.0 mL of
a strong acid at pH 3.0 and 3.0 mL of a strong base
at pH 10.
Solution
First, find the concentration of the acid and the base
pH = 3.0 means [H+] = 10-3.0
pH = 10 means [H+] = 10-10 M or [OH-] = 10-4 M
Now find the number of mmol of each
mmol H+ = 10-3 x 2.0 = 2.0x10-3 mmol
mmol OH- = 10-4 x 3.0 = 3.0 x 10-4 mmol
mmol H+ excess = 2.0x10-3 – 3.0x10-4 = 1.7x10-3
[H+] = 1.7x10-3/5 = 3.4x10-4 M
pH = - log 3.4x10-4 = 3.5](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-7-320.jpg)


![10
For the conjugate base of acetic acid (acetate)
we have
OAc- + H2O D HOAc + OH-
Kb = [HOAc][OH-]/[OAc-]
Let us multiply ka times kb we get
Ka kb = [H+][OH-] = kw , or
Ka kb = kw
Therefore, if we know the ka for the acid we can
get the equilibrium constant for its conjugate
base since we know kw. We can find ka for
the conjugate acid by the knowledge of the
equilibrium constant of the parent base.](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-10-320.jpg)

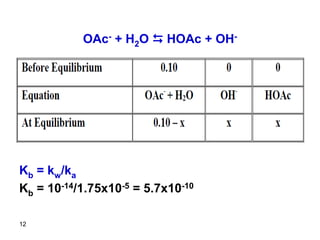
![13
Kb = [HOAc][OH-]/[OAc-]
Kb = x * x/(0.10 – x)
Kb is very small and we can fairly assume that
0.10>>x
5.7x10-10 = x2/0.1
x = 7.6 x 10-6
Relative error = (7.6x10-6/0.10) x100 = 7.6x10-3%
The assumption is valid.
[OH-] = 7.6x10-6 M
[H+] = 1.3x10-9 M = [OH-]water
pOH = 5.12
pH = 14 – 5.12 = 8.88](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-13-320.jpg)
![14
therefore we can write
[H+]solution = [H+]ammonium
The first point is to write the equilibrium where
NH4
+ D H+ + NH3
Calculate the pH of a 0.25 M ammonium chloride solution. Kb =
1.75x10-5](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-14-320.jpg)
![15
Ka = 10-14/1.75x10-5 = 5.7x10-10
Ka = [H+][NH3]/[NH4
+]
Ka = x * x / (0.25 – x)
Ka is very small. Assume 0.25 >> x
5.7*10-10 = x2/0.25
x = 1.2x10-5
Relative error = (1.2x10-5/0.25) x 100 = 4.8x10-3 %
The assumption is valid and the [H+] = 1.2x10-5 M
pH = 4.92](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-15-320.jpg)

![18
It is therefore justified to assume that the only
important equilibrium is:
CN- + H2O D HCN + OH- kb = 1.7*10-5
0
0
Before equil
OH-
HCN
CN- + H2O
Equation
x
x
0.1 - x
After equil
Kb = [HCN][OH-]/[CN-]](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-18-320.jpg)
![19
1.7*10-5 = x * x / (0.10 – x)
kb is very small that we can assume that
0.10>>x. We then have:
1.8*10-5 = x2 / 0.1
x = 1.3x10-3 M
Relative error = (1.3x10-3 /0.1) x 100 = 1.3%
The assumption is valid, therefore:
[OH-] = 1.3x10-3 M
pOH = 2.89
pH = 11.11](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-19-320.jpg)

![21
Hasselbalch-Henderson Equation
In the acetic acid/acetate buffer described
above, we have:
Ka = [OAc-][H+]/[HOAc]
Pka = pH – log [OAc-]/[HOAc]
pH = pka + log [OAc-]/[HOAc]
The above equation is referred to as
Hasselbalch-Henderson equation.](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-21-320.jpg)

![24
Calculate the pH of the buffer solution
prepared by mixing 10 mL 0.10 M HOAc (ka =
1.75x10-5) with 20 mL of 0.20 M sodium
acetate.
Solution
Let us first calculate the concentrations after
mixing (final concentrations of the acid and
its conjugate base)
mmol HOAc = 0.10 x 10 = 1.0 mmol
[HOAc] = 1.0/30
mmol OAc- = 0.20 x 20 = 4.0 mmol](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-24-320.jpg)
![25
[OAc-] = 4.0/30
pH = 5.36 using Hasselbalch-Henderson Equation](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-25-320.jpg)

![27
Now the mmol base added will react in a 1:1 mole
ratio with the acid . Therefore we have
mmol HOAc left = 6.0 – 2.5 = 3.5 mmol
[HOAc] = 3.5/55 M
mmol OAc- formed = 2.5 mmol
[OAc-] = 2.5/55 M
pH = 4.61 using Hasselbalch-Henderson Equation](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-27-320.jpg)

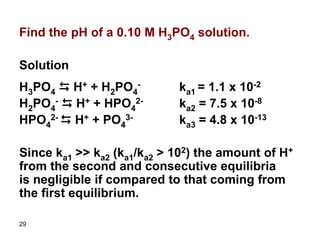
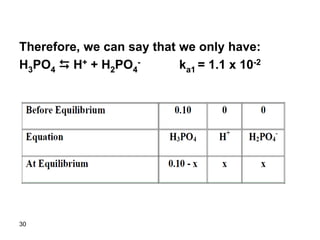
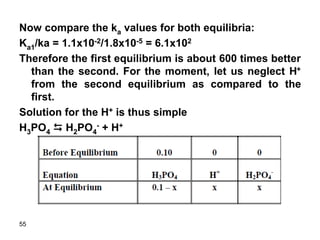
![31
Ka1 = x * x/(0.10 – x)
Assume 0.10>>x since ka1 is small (!!!)
1.1*10-2 = x2/0.10
x = 0.033
Relative error = (0.033/0.10) x 100 = 33%
The assumption is invalid and thus we have to
use the quadratic equation. If we solve the
quadratic equation we get:
X = 0.028
Therefore, [H+] = 0.028 M
pH = 1.55](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-31-320.jpg)

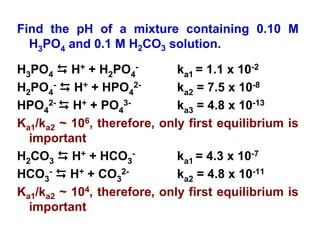
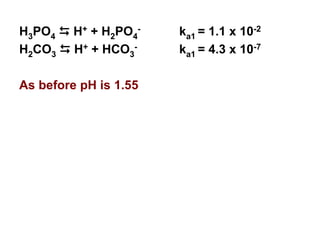
![33
H2CO3 D H+ + HCO3
- ka1 = 4.3 x 10-7
Ka1 = x * x/(0.10 – x)
Assume 0.10>>x since ka1 is small
4.3*10-7= x2/0.10
x = 2.1x10-4
Relative error = (2.1x10-4/0.10) x 100 = 0.21%
The assumption is valid and [H+] = 2.1x10-4 M
pH = 3.68](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-33-320.jpg)
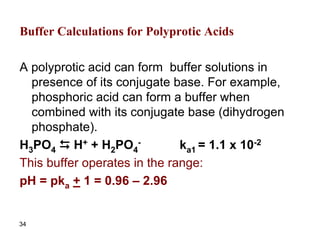
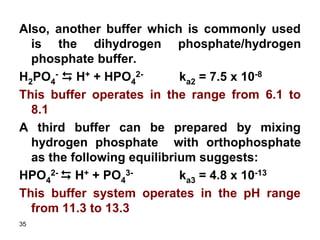

![37
Find the ratio of [H2PO4
-]/[HPO4
2-] if the pH of
the solution containing a mixture of both
substances is 7.4. ka2 = 7.5x10-8
Solution
The equilibrium equation combining the two
species is:
H2PO4
- D H+ + HPO4
2- ka2 = 7.5 x 10-8
Ka2 = [H+][HPO4
2-]/[H2PO4
-]
[H+] = 10-7.4 = 4x10-8 M
7.5x10-8 = 4x10-8 [HPO4
2-]/[H2PO4
-]
[HPO4
2-]/[H2PO4
-] = 1.9](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-37-320.jpg)

![HOAc D H+ + OAc-
We can now find the concentration of the acid
where:
1.8*10-5 = 10-5*0.2/[HOAc]
[HOAc] = 0.2/1.8 = 0.11 M
mmol HOAc = 0.11*500 = 55.6
12.0*VHOAc = 55.6
VHOAc = 4.6 mL
39](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-39-320.jpg)


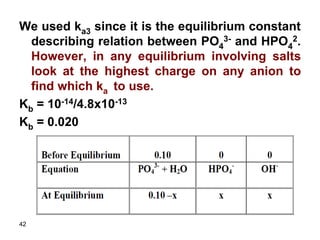
![43
Kb = x * x/0.10 – x
Assume 0.10 >> x
0.02 = x2/0.10
x = 0.045
Relative error = (0.045/0.10) x 100 = 45%
Therefore, assumption is invalid and we have
to use the quadratic equation. If we solve the
quadratic equation we get:
X = 0.036
Therefore, [OH-] = 0.036 M
pOH = 1.44 and pH = 14 – 1.44 = 12.56](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-43-320.jpg)
![44
2. Protonated Salts
These are usually amphoteric salts which
react as acids and bases. For example,
NaH2PO4 in water would show the following
equilibria:
H2PO4
- D H+ + HPO4
2-
H2PO4
- + H2O D OH- + H3PO4
H2O D H+ + OH-
[H+]solution = [H+]H2PO4
- + [H+]H2O – [OH-]H2PO4
-
[H+]solution = [HPO4
2-] + [OH-] – [H3PO4]](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-44-320.jpg)
![45
Now make all terms as functions in either H+ or H2PO4
-, then we have:
[H+] = {ka2[H2PO4
-]/[H+]} + kw/[H+] – {[H2PO4
-][H+]/ka1}
Rearrangement gives
[H+] = {(ka1kw + ka1ka2[H2PO4
-])/(ka1 + [H2PO4
-]}1/2
At high salt concentration and low ka1 this relation may be approximated to:
[H+] = {ka1ka2}1/2
Where; the pH will be independent on salt
concentration but only on the equilibrium
constants.](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-45-320.jpg)
![46
Protonated Salts with multiple charges
HPO4
2- is a protonated salt which behaves as an
amphoteric substance where the following equilibria
takes place:
HPO4
2- D H+ + PO4
3-
HPO4
2- + H2O D H2PO4
- + OH-
H2O D H+ + OH-
[H+] = [H+]HPO4
- + [H+]water – [OH-]HPO4
-
[H+] = [PO4
3-] + [OH-] – [H2PO4
-]
[H+] = ka3 [HPO4
2-]/[H+] + kw/[H+] – [H+][HPO4
2-]/ka2
Rearrangement of this relation gives
[H+] = {(ka2kw + ka2ka3 [HPO4
2-])/(ka2 + [HPO4
2-])}1/2
Approximation, if valid, gives:
[H+] = (ka2ka3)1/2](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-46-320.jpg)
![47
Find the pH of a 0.10 M NaHCO3 solution. ka1 = 4.3
x 10-7, ka2 = 4.8 x 10-1
[H+] = {ka1ka2}1/2
[H+] = {4.3x10-7 * 4.8x10-11}1/2 = 4.5x10-9 M
pH is 8.34
This is since the salt concentration is high
enough. Now look at the following example
and compare:](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-47-320.jpg)
![48
Find the pH of a 10-3 M NaHCO3 solution. ka1 =
4.3 x 10-7, ka2 = 4.8 x 10-1
Substitution in the relation [H+] = {ka1ka2}1/2 will
give pH 8.34 not accurate
Accurate
pH = 8.10](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-48-320.jpg)
![49
Find the pH of a 0.20 M Na2HPO4 solution. Ka1 =
1.1x10-2, ka2 = 7.5x10-8, ka3 = 4.8x10-13.
Using the approximated expression we get:
[H+] = (7.5x10-8 * 4.8x10-13)1/2 = 1.9x10-10 M
pH = 9.72](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-49-320.jpg)

![51
Find the pH of a solution containing 0.10 M HCl
and 0.10 M HNO3.
Solution
[H+] = [H+]HCl + [H+]HNO3
Both are strong acids which are 100%
dissociated. Therefore, we have
[H+] = 0.10 + 0.10 = 0.2
pH = 0.70](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-51-320.jpg)
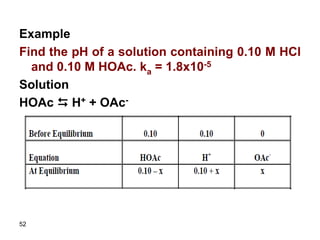
![53
Ka = (0.10 + x) x/(0.10 – x)
Assume 0.1 >> x since ka is small
1.8x10-5 = 0.10 x/0.10
x = 1.8x10-5
Relative error = (1.8x10-5/0.10) x100 = 1.8x10-2%
Therefore [H+] = 0.10 + 1.8x10-5 = 0.10
pH is 1
It is clear that all H+ comes from the strong
acid since dissociation of the weak acid is
limited and in presence of strong acid the
dissociation of the weak acid is further
suppressed.](https://image.slidesharecdn.com/acid-base-equilibria-230417211608-5113c093/85/Acid-Base-Equilibria-ppt-53-320.jpg)