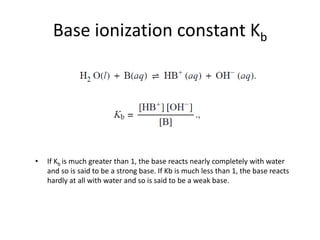
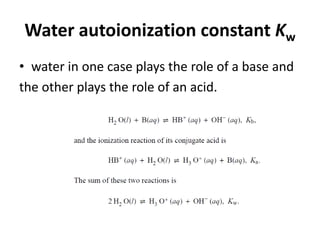
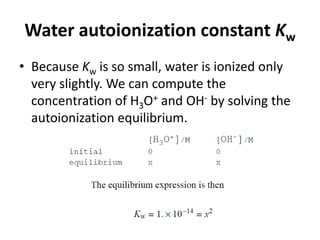
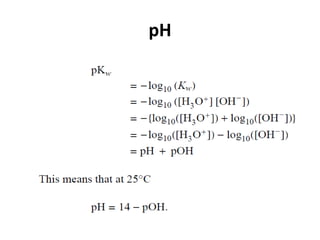
This document discusses acid-base concepts including:
- Arrhenius and Bronsted-Lowry definitions of acids and bases
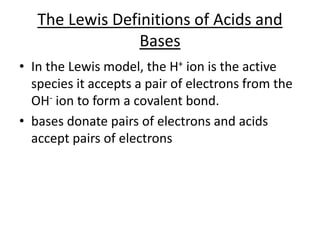
- Conjugate acid-base pairs and the Lewis definitions
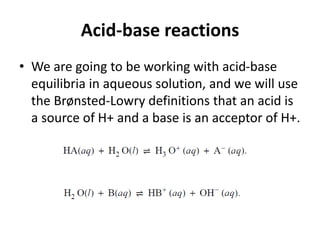
- Acid-base reactions and the Brønsted-Lowry definitions of acids donating H+ and bases accepting H+
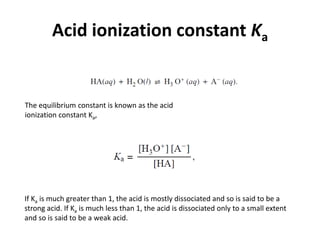
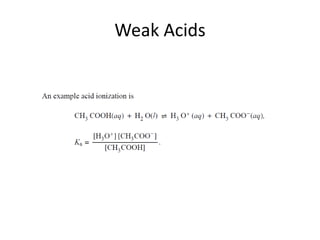
- Acid ionization constants (Ka) and base ionization constants (Kb) as well as the water autoionization constant (Kw) and how it relates to pH
- Calculations involving pH, pOH, [H+], Ka, Kb, and the Henderson-Hasselbalch equation for buffer solutions




![[H+] pH
10-7 = 7
10-3 = 3
10-2 = 2
10-10 = 10
5x10-4 = 3.3
7x10-6 = 5.15
3.3x10-8 = 7.48
pH = -Log[H+]
It is easier to think in log
of concentrations but it
takes practice!!](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-14-320.jpg)
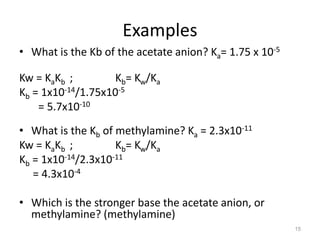
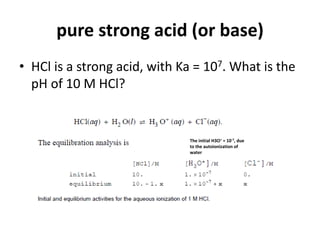
![PH of strong acids and bases
What is the pH of a 0.5 x10-7 M KOH solution?
KOH → K+ + OH-
Before dissociation 0.5 x10-7 M 0 0
After dissociation0 0 0.5 x10-7 0.5 x10-7 M
because it completely dissociates, so
[OH-] = 1x10-7 (from water) + 0.5 x10-7(from the base)
= 1.5 x10-7 M
pOH= -log (1.5 x10-7) = 6.82
pH = 14 - 6.82
= 7.18
17
In a solution strong acid that are less concentrated
than 1 * 10-6 M, is safer to assume that the
equilibrium concentration of H3O+ is equal to the
molar concentration of the acid + the H3O+
concentration from water.
[H+] = [H+] (from water) + [H+] (from the acid)
The same is true for the hydroxide concentration in
solution of strong base](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-17-320.jpg)
![PH of strong acids and bases
What is the pH of a 5.4x10-3 M solution of NaOH?
NaOH → Na+ + OH-
Before dissociation 5.4x10-3 M o 0
After dissociation 0 5.4x10-3 5.4x10-3 M
because it completely dissociates, so
[NaOH] = 0, [Na+] = 5.4x10-3, [OH-] = 5.4x10-3
pOH = -log 5.4x10-3 = 2.3
pH= 14- pOH
= 11.7
18](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-18-320.jpg)

![Answer
Ka= [H+][A-]/[HA]
HA ⇌ H+ + A-
Keq= [H+][A-]/[HA]
6.8 *10-4 = (x)(x) / (0.05)
X2= 6.8 *10-4 * 0.05
X= [H+]=[A-]= 0.00583
pH= -log[H+]= -log (0.00583) = 2.23
Initial 0.05 0 0
Equilibrium 0.05-x x x
After
Neglectance 0.05 x x
20](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-20-320.jpg)

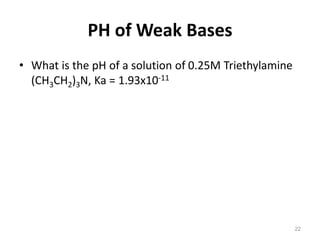
![Answer
Kw= Ka * Kb Kb= Kw/Ka= 1.0 * 10 -14 / 1.93x10-11 = 5.18 * 10-4
Kb= [BH+][OH-]/[B]
B + H2O ⇌ BH+ + OH-
Kb= [BH+][OH-]/[B]
5.18 * 10-4 = (x)(x) / (0.25)
X2= 5.18 * 10-4 * 0.25
X= [BH+]= [OH-]= 0.0114
pOH= -log[OH-]-log (0.0114) = 1.94 pH= 14 - 1.94= 12.06
Initial 0.25 0 0
Equilibrium 0.25-x x x
Neglect 0.25 x x
23](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-23-320.jpg)
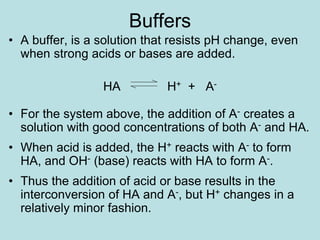
![If you establish equilibrium, changes in [H+] will shift
the ratio of HA and A-.
By adding more H+ , A- will be consumed
forming HA.
If there is sufficient [A-], the extra H+ will also be
consumed and the [H+] will not change.
A
H
HA](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-25-320.jpg)

![Henderson - Hasselbalch equation
]
[
]
][
[
HA
A
H
K
From
]
[
]
[
]
[
A
HA
K
H
Rearrange
Take (-)Log of each
]
[
]
[
log
log
HA
A
K
pH
]
[
]
[
log
HA
A
pK
pH
](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-29-320.jpg)
![Above and below this range there is insufficient
amount of conjugate acid or base to combine with
the base or acid to prevent the change in pH.
[HA]
]
[A
log
pK
pH
-
1
10
10
1
from
varies
ratio
]
HA
[
]
[A
](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-30-320.jpg)
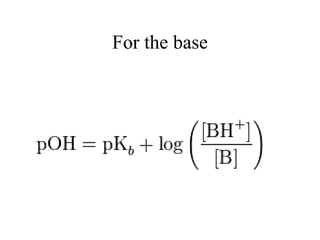
![For weak acids
HA A- + H+
This equilibrium depends on concentrations of each component
.
If [HA] = [A-] or 1/2 dissociated
Then 0
1
log
]
[
]
[
log
HA
A
By definition the pK is the pH where [HA] = [A-] :
50% dissociated
: pH = pK](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-31-320.jpg)
![You will be asked to solve Henderson -
Hasselbalch type problems:
You may be asked the pH, pK, the ratio of acid or base or solve for
the final concentrations of each.
also note : when [A-] > [HA] pH>pKa
when [A-] < [HA] pH <pKa
Exams Problems
[HA]
]
[A
log
pK
pH
-
](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-32-320.jpg)

![What is the pH of a solution of that contains 0.1M CH3C00-
and 0.9 M CH3C00H?
1) pH = pK + Log [A-]
[HA]
2) CH3C00H CH3C00- + H+
3) Find pH
4) pK = 4.76 A- = 0.1 M HA = 0.9 M
5) Already at equilibrium
6) X = 4.76 + Log 0.1
0.9
Log 0.111 = -.95 X = 4.76 + (-.95) X = 3.81](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-34-320.jpg)
![What would the concentration of CH3C00- be at pH 5.3
if 0.1M CH3C00H was adjusted to that pH.
1) pH = pK + Log [A-]
[HA]
2) CH3C00H CH3C00- + H+
3) Find equilibrium value of [A-] i.e [CH3C00-]
4) pH = 5.3; pK = 4.76
5) Let X = amount of CH3C00H dissociated at equilibrium
[A-] = [X]
[HA] = [0.1 - X]
6) 5.3 = 4.76 + Log [X]
[0.1 - X]
Now solve.](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-35-320.jpg)

![Answer
Salt CH3COONa → Na+
+ CH3COO-
Cacetate
Acid CH3COOH ⇌ H+
+ CH3COO-
Initial CHA Cacetate
Equilibrium CHA - x x Cacetate + x
neglect CHA x Cacetate
Salt
Mol of Acetate = 0.05 L * 0.5 M = 0.025
Cacetate= mol / voltotal = 0.025 mol / (0.05 L+ 0.025 L) = 0.333 m
Acid
Mol of Acetic acid = 0.025 L * 0.1 M = 0.0025
CHA= mol / voltotal = 0.0025 mol / (0.05 L+ 0.025 L) = 0.0333 m
pH= pKa + log ([A-]/[HA]) pH= 4.757 + log ([0.333]/[0.0333])
pH= 5.757
A-
Simply, you can use the dilution law
C1V1=C2V2
37](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-37-320.jpg)
![Answer
We can solve the problem by another way
Ka= [H+][CH3COO-]/[CH3COOH]
1.8 *10-5 = [H+][ 0.333] / 0.0333
[H+]= 1.8 *10-5 * 0.0333 / 0.333
[H+]= 1.8 *10-6
pH= 5.757
38](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-38-320.jpg)
![Tolerance of a Buffer System
• If we need to check the stability of a buffer
system for any minor change in the buffer
acidity, basicity or system volume, then we
will examine the following cases.
• After calculation of the final concentration of
[HA] and [A-] after addition of acid or base
then the pH can be calculated by using
Henderson-Hasselbalch equation:
pH= pKa + log ([A-]/[HA])
39](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-39-320.jpg)
![Tolerance of a Buffer System:
Case#1
• Case #1
Start with 1 L of pure water and add 5 mL of 10M HCl. What would
be the initial and final pH?
• The initial pH for pure water = 7
• For the dilution of 5 mL of 10M HCl in 1 L of water, the final
volume of the solution will be 1.005 L.
C1V1=C2V2→ 10 M * 0.005 L = C2 * 1.005 L
C2 = 0.04975 M the concentration of HCl after dilution
HCl → H+ + Cl-
0.04975 mol /L 0.04975 mol /L 0.04975 mol /L
pH = -log [H+] = -log [0.04975 M]= 1.3
So the pH of water had been dropped from 7 to 1.3 by addition of 5 mL
of 10 M HCl 40](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-40-320.jpg)
![Tolerance of a Buffer System:
Case#2
• Case #2
In comparison with Case#1.
For 1 L buffer solution of 0.364 M weak
acid [HA] and 0.636 M [A-] calculate the
initial and final pH after addition of 5 mL of
10 M HCl. Ka= 1.8 *10-5 (pKa= 4.757)
41](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-41-320.jpg)
![Tolerance of a Buffer System:
Case#2
• The initial pH for the buffer system can be
calculated using Henderson-Hasselbalch equation
pH= pKa + log ([A-]/[HA])
pH= 4.757 + log (0.636 M/ 0.364 M)
pH = 4.757 + 0.242 = 5
[H+] = 10-pH = 1.00 * 10-5
The [H+] can be calculated by another way
HA ⇌ H+ + A-
0.364 x 0.636
Ka= [H+][A-] /[HA]
[H+]= Ka * [HA] / [A-] = 1.8 *10-5 * 0.364 / 0.636
[H+]= 1.03 *10-5
pH = -log [H+]= -log [1.03 *10-5 ] = 5 42](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-42-320.jpg)
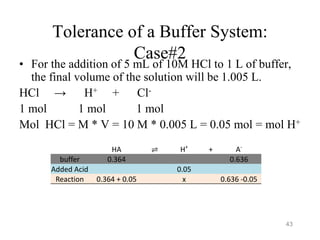
![Tolerance of a Buffer System:
Case#2
The total volume of the solution after addition of 5 mL of 10 M HCl to 1 L of buffer =
1.005 L
Mol A- = 0.636 – 0.05 = 0.586
[A-] = 0.586 mol / 1.005 L = 0.583 M
Mol HA = 0.364 + 0.05 = 0.414
[HA] = 0.414 mol / 1.005 L = 0.412 M
pH= pKa + log ([A-]/[HA])
pH = 4.757 + log (0.583 / 0.412)= 4.90
Therefore, the pH of the buffer system has dropped by about 0.1 ( from 5.00 to 4.90)
In comparison with water, the buffer solution seems to be more resistance to the
addition of acid.
44](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-44-320.jpg)
![Fraction Dissociation
Fraction Dissociation = [A-]/total acid (all forms)
= [A-]/ ([HA] + [A-])
If [A-]= 0.0055
Total acid = [A-] + [HA] = F acid = 0.05
Fraction dissociation = 0.0055/0.05= 0.11 or 11%
From fraction dissociation determine Ka (if an acid is 1%
dissociated when the formal concentration is 0.1M, what is the
Ka?)
From pH at a given [ ] of weak acid determine the Ka (if the pH of
a 0.1M solution of acid is 2, what is the Ka of the acid?)
46](https://image.slidesharecdn.com/acid-base-equilebria-221208110343-3f2308e0/85/acid-base-equilebria-ppt-46-320.jpg)