This document outlines qualitative tests for carbon, hydrogen, oxygen, nitrogen, halogens, and sulfur in organic compounds. Carbon and hydrogen are detected by heating compounds with CuO and observing the formation of calcium carbonate and water droplets. Oxygen is detected using ferrox paper or iron(III) hexathiocyanatoferrate(III) solutions, which turn red in the presence of oxygen. Nitrogen, halogens, and sulfur require sodium fusion to convert them to inorganic ions before qualitative testing, such as using Prussian blue for nitrogen or lead sulfide for sulfur.


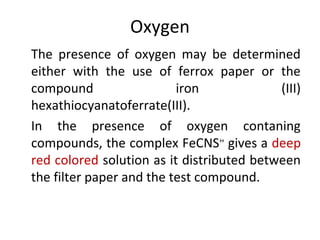

![•
2Fe(NH4)(SO4)2 + 6 KSCN
Iron (III) ammonium sulfate potassium thiocyanate
Fe[Fe(SCN)6] + 3 K2SO4 + (NH4)2SO4
iron (III) hexathiocyanatoferrate(III)](https://image.slidesharecdn.com/qualitativetestsforelementsinorganiccompounds-160303011052/85/Qualitative-tests-for-elements-in-organic-compounds-5-320.jpg)

![•
Organic compound [C] [H][O][N][X][S] + N
fusion
NaCN, NaOH, NaX and Na2S
The resulting organic compounds are tested
qualitatively for the presence of N, S and
halogens.](https://image.slidesharecdn.com/qualitativetestsforelementsinorganiccompounds-160303011052/85/Qualitative-tests-for-elements-in-organic-compounds-7-320.jpg)
![NITROGEN
•
Detected by the formation of Prussian blue
after the sodium fusion.
•
Nitrogen in cyanide form is converted to
sodium ferrocyanide, which produces Prussian
blue (ferric ferrocyanide, Fe4[Fe(CN)6]3 with
ferric chloride in acid solution.
•
Nitrogen in the form of amino nitrogen (-NH2),
heating with soda lime (a mixture of fused
NaOH and CaO) will liberate ammonia gas,
which is tested with moist red litmus paper.](https://image.slidesharecdn.com/qualitativetestsforelementsinorganiccompounds-160303011052/85/Qualitative-tests-for-elements-in-organic-compounds-8-320.jpg)


