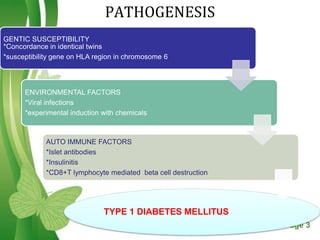
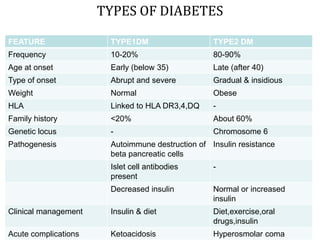
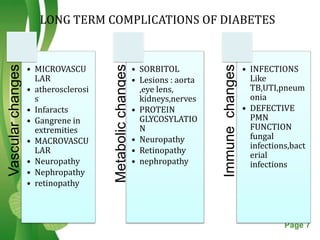
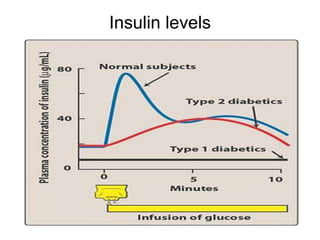
This document discusses oral hypoglycemic drugs and insulin used to treat diabetes. It describes the two main types of diabetes - type 1 caused by insulin deficiency and type 2 caused by insulin resistance. The document outlines several classes of oral hypoglycemic drugs including biguanides, sulfonylureas, meglitinides, thiazolidinediones, and alpha-glucosidase inhibitors. It provides details on the mechanism of action, pharmacokinetics, effects and side effects of drugs from each class.