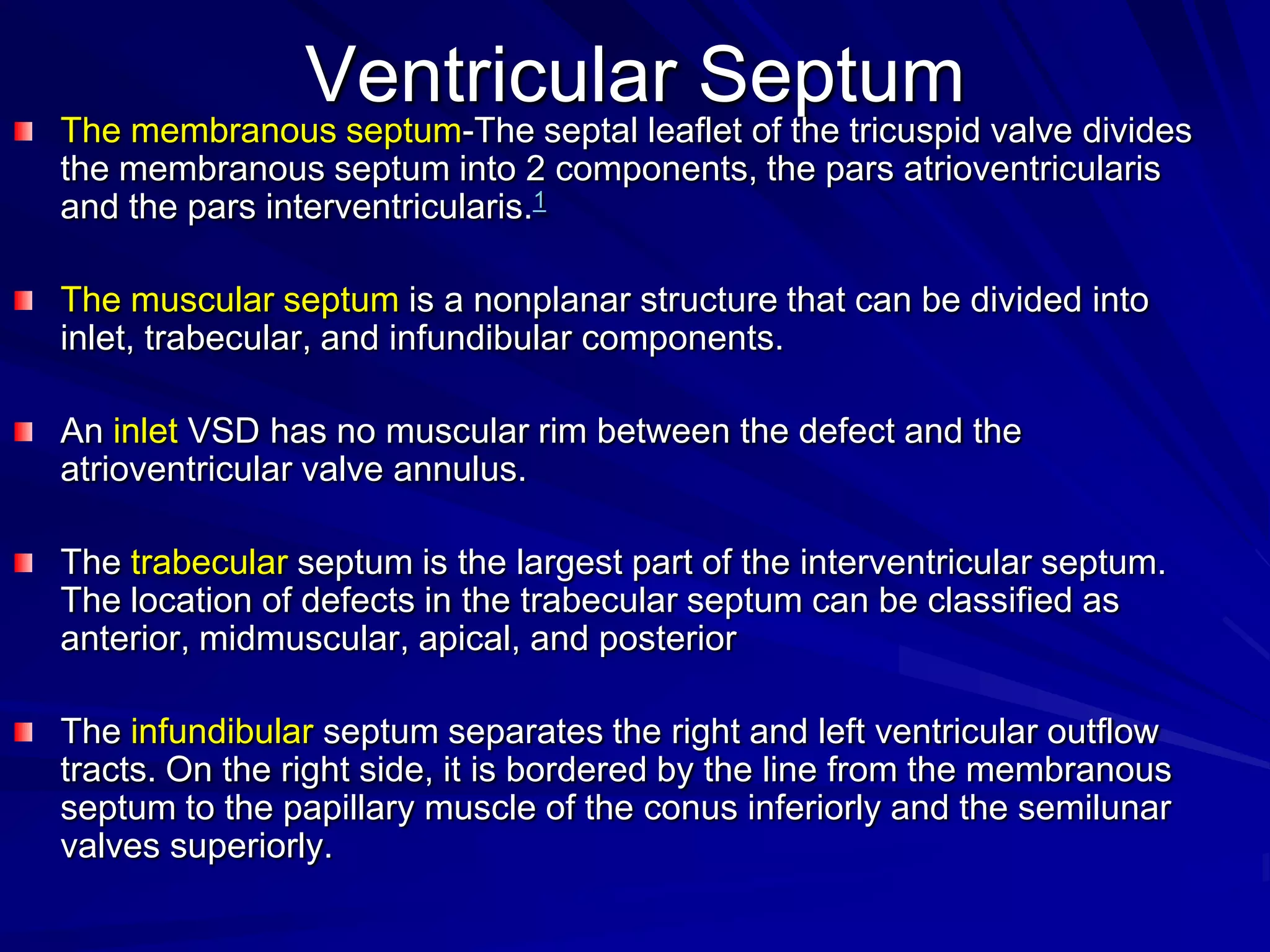
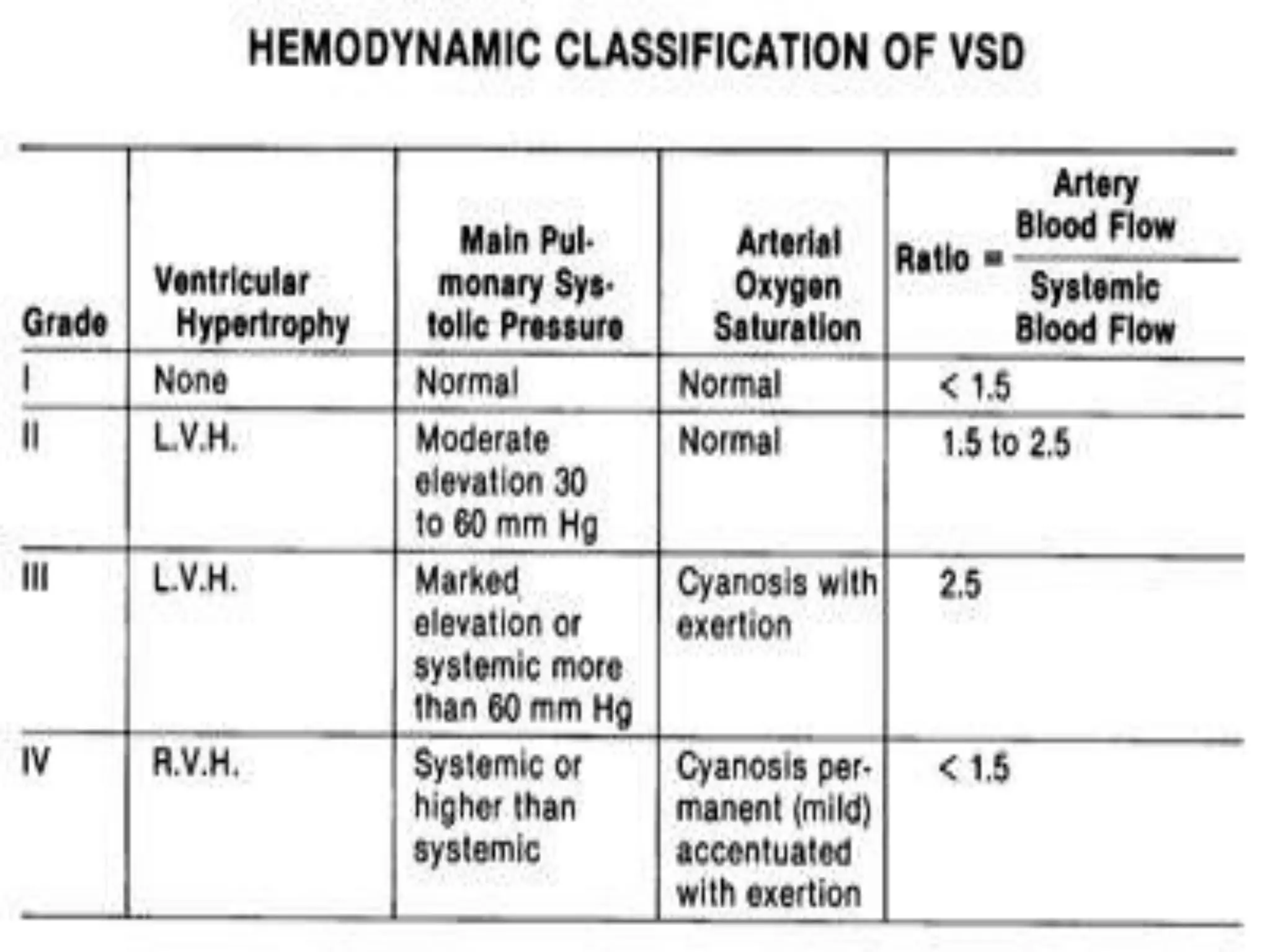
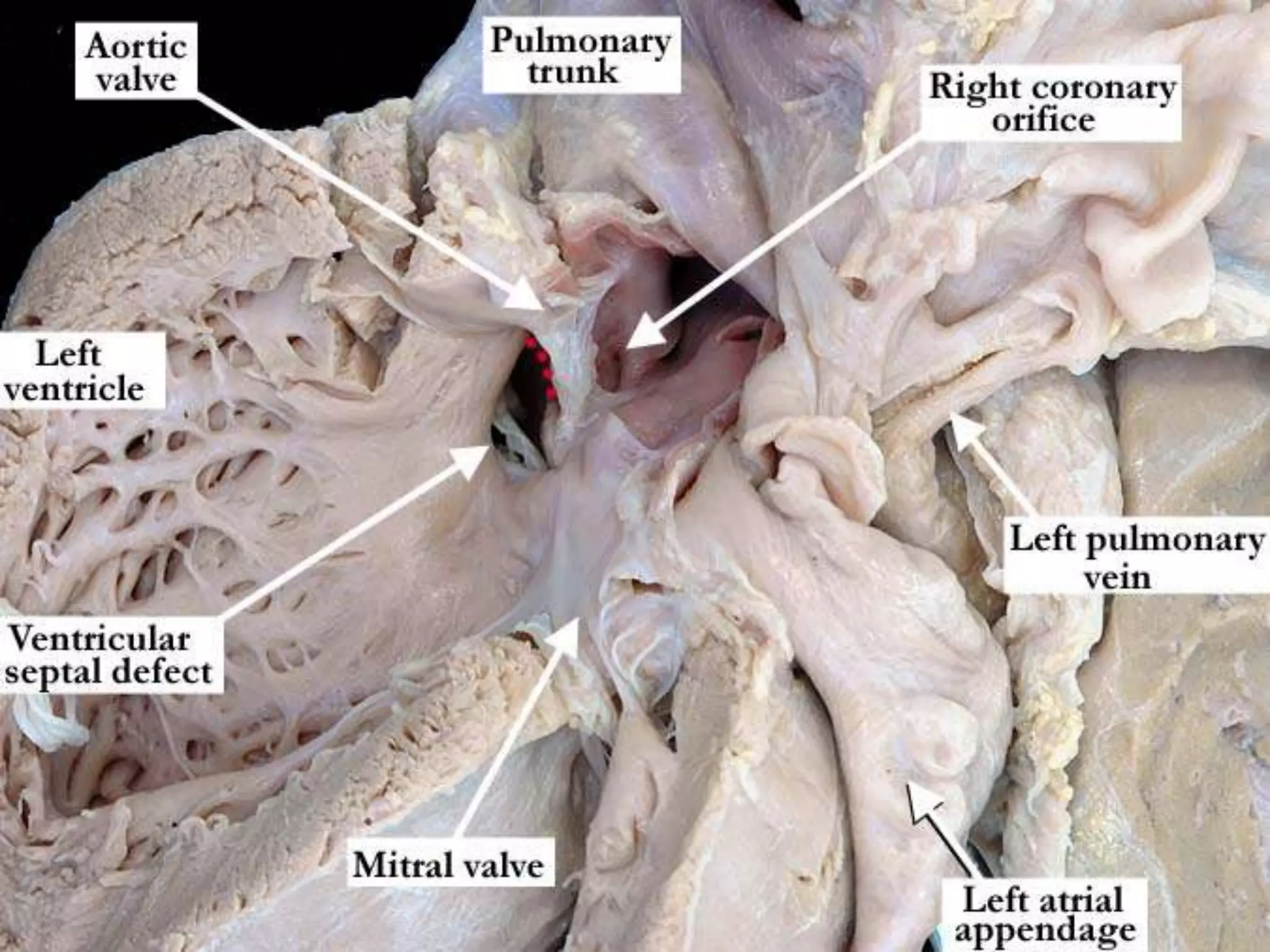
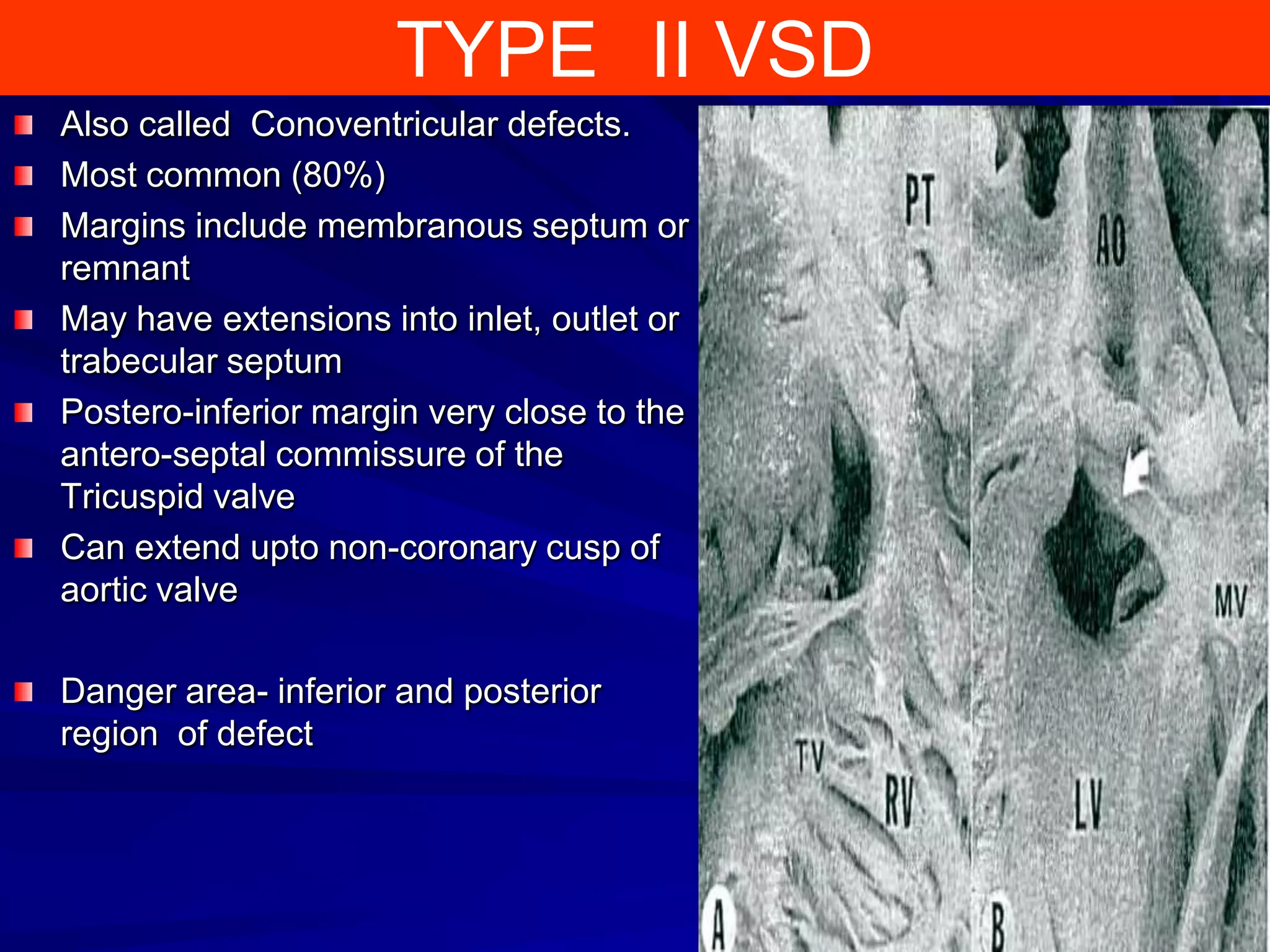
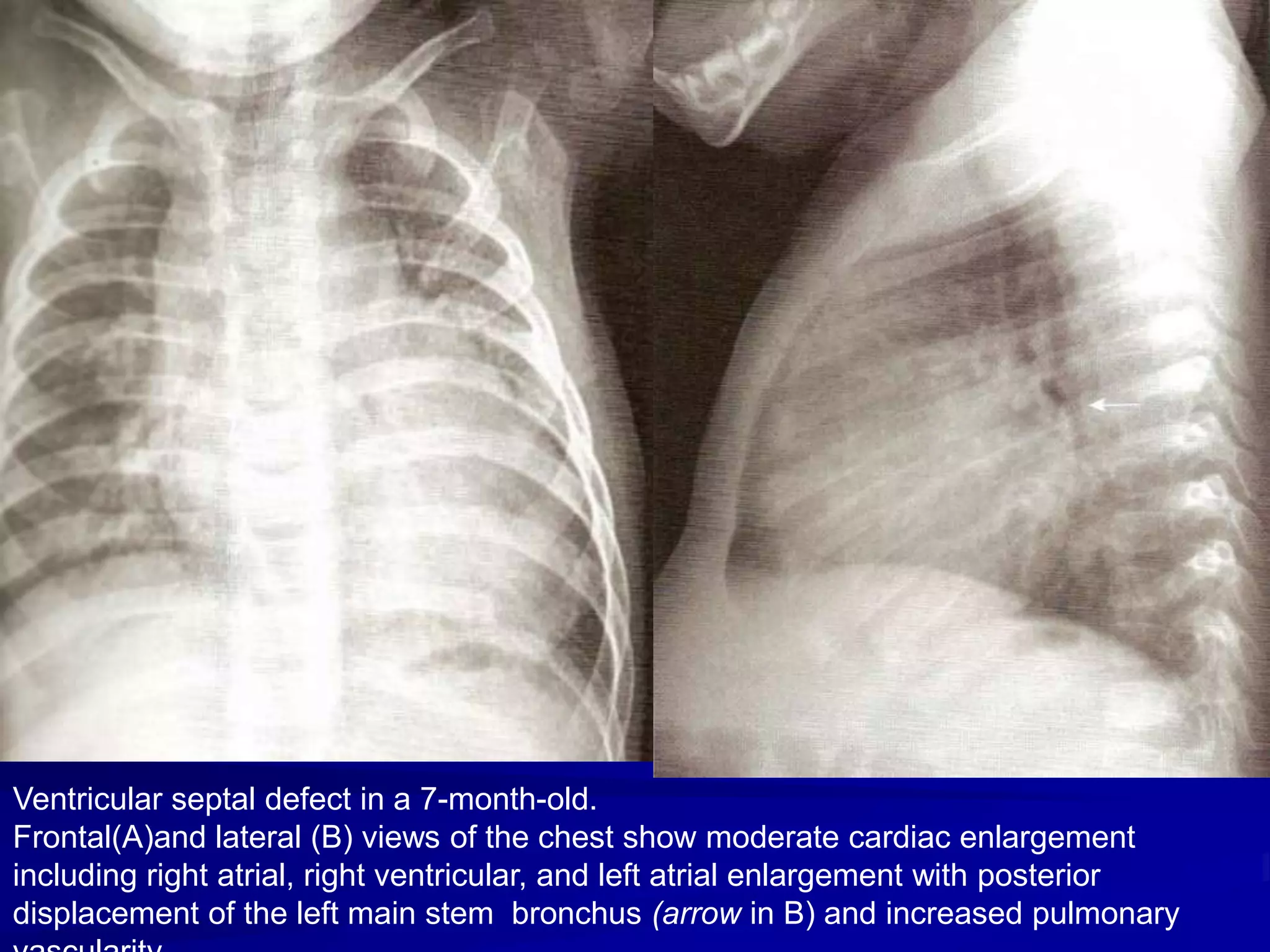
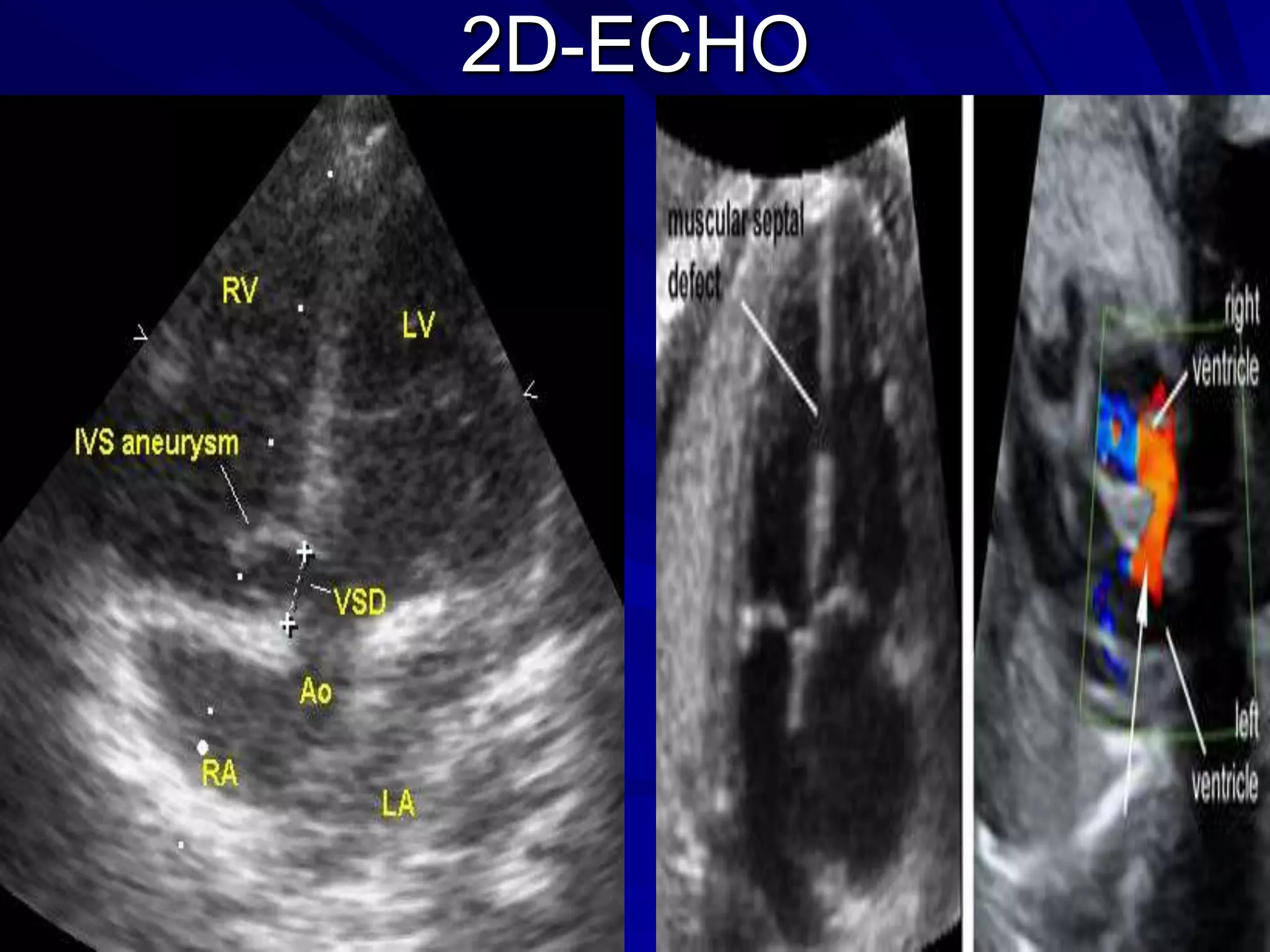
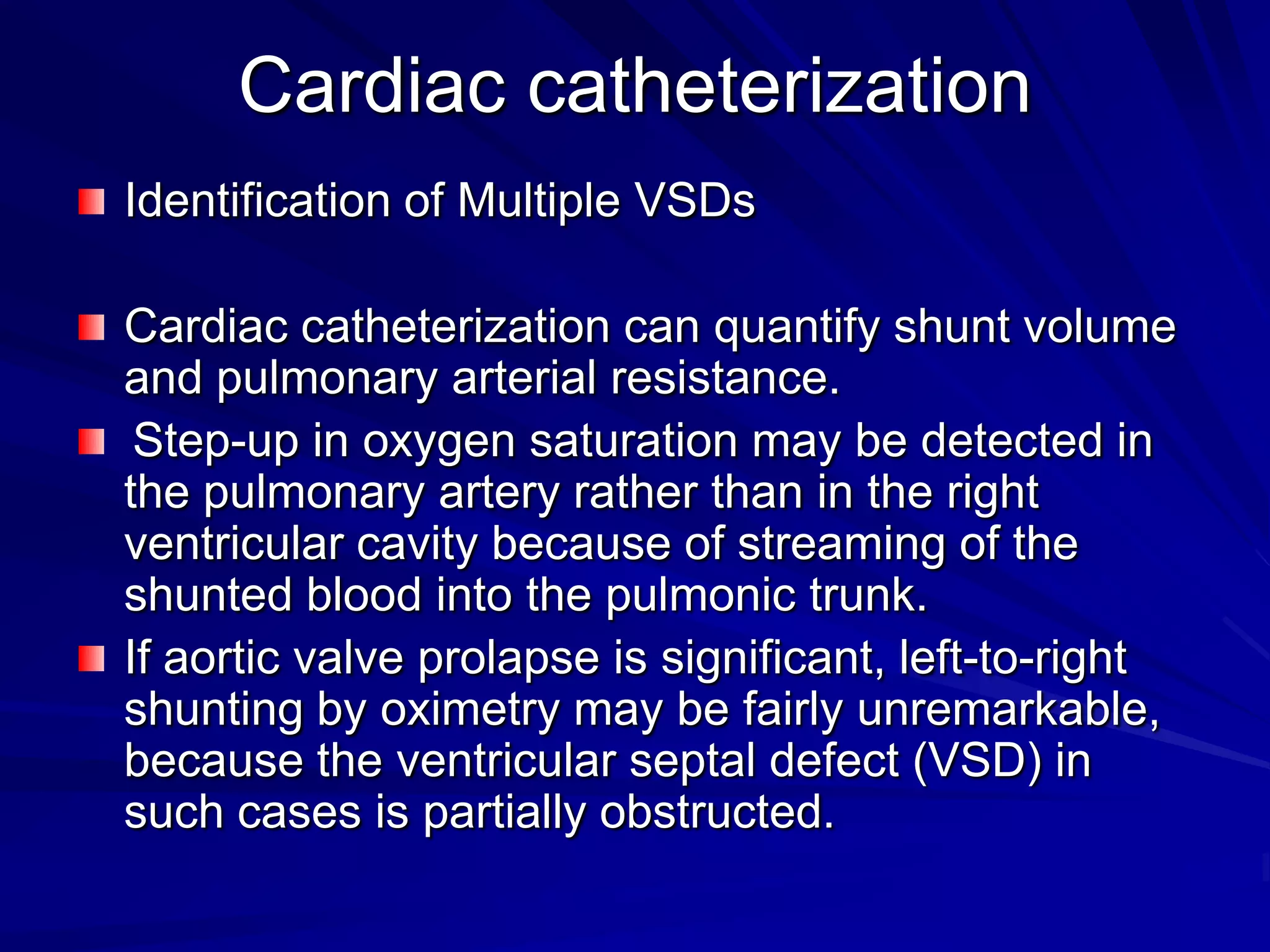
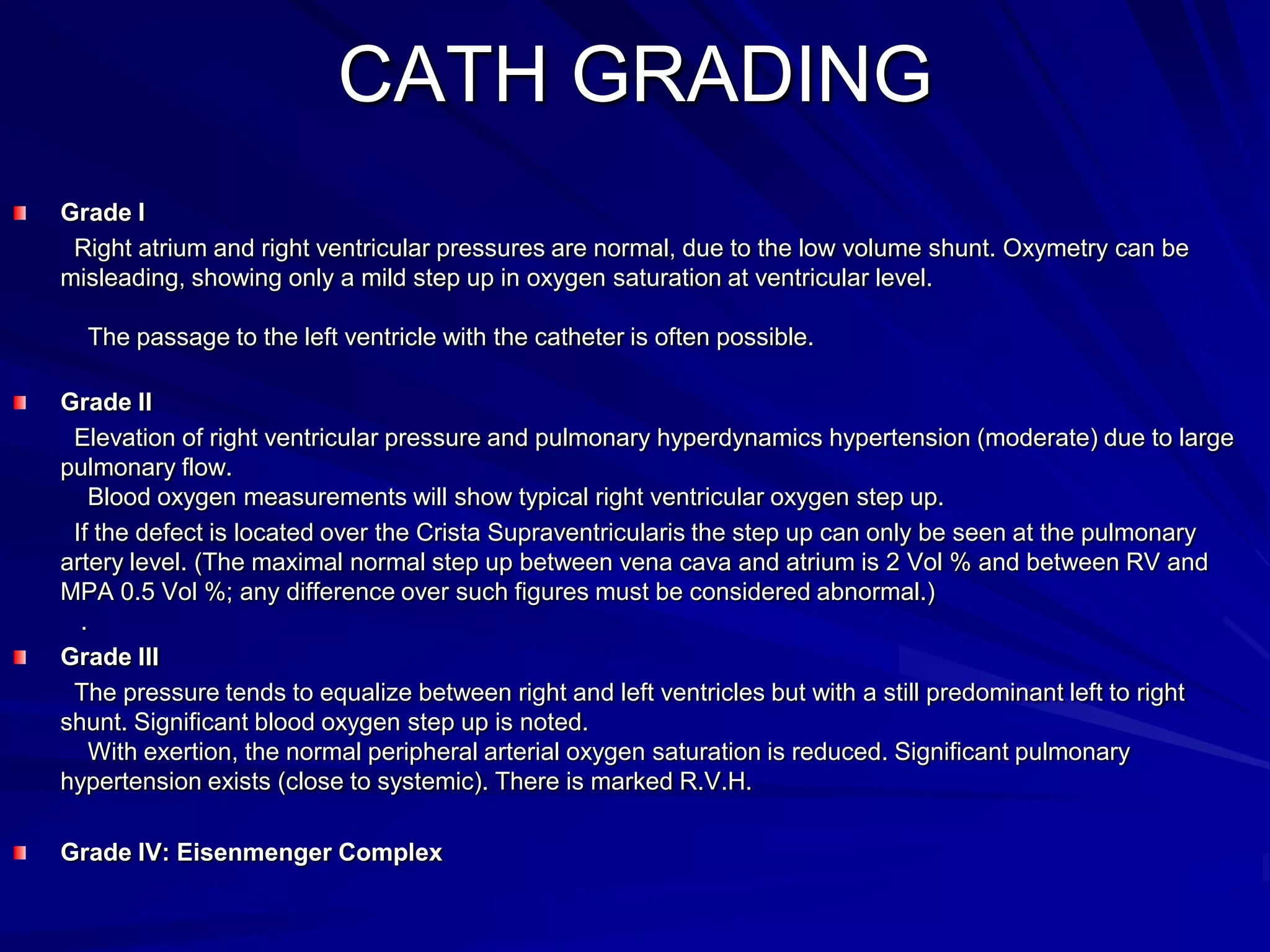
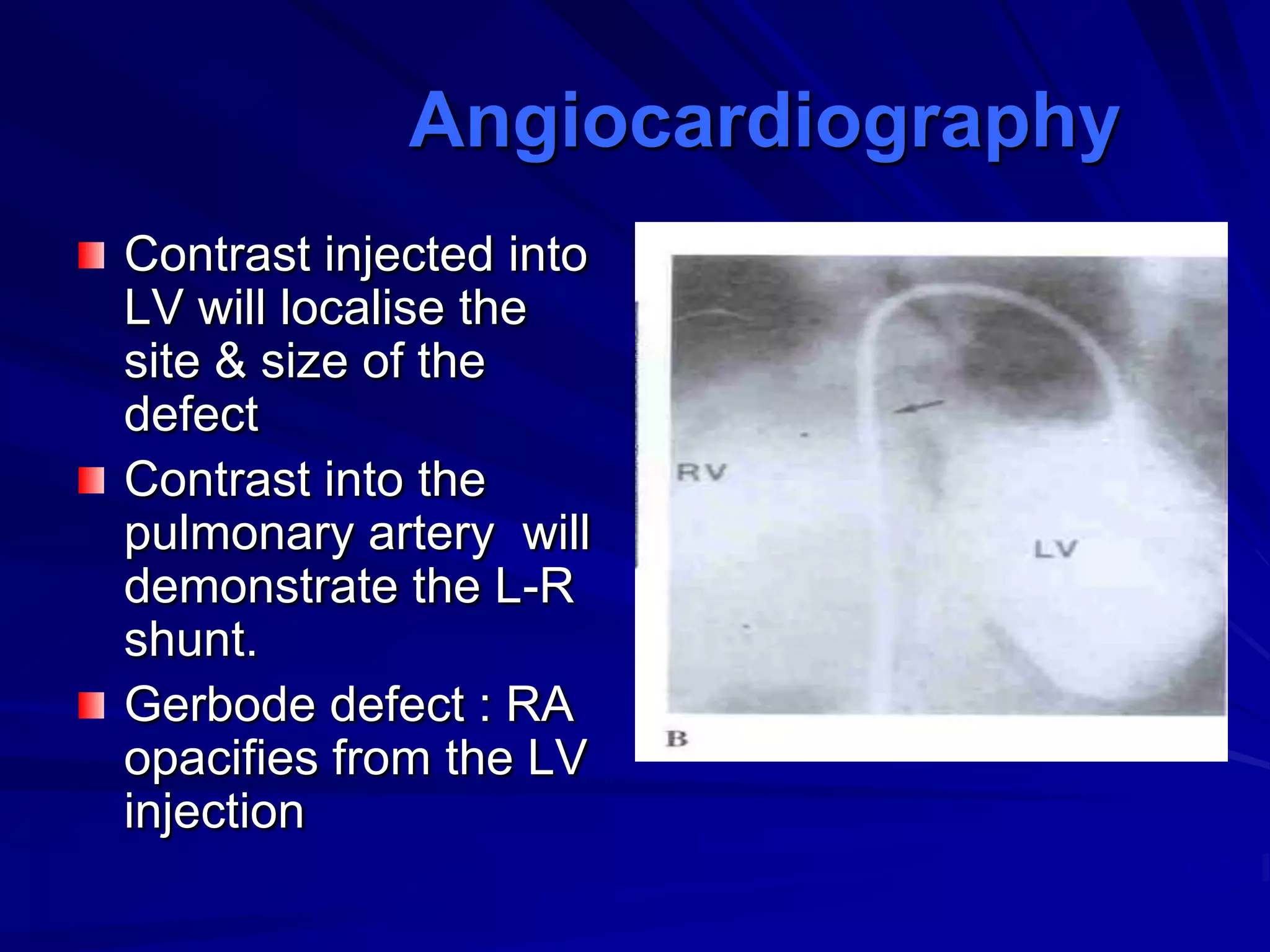
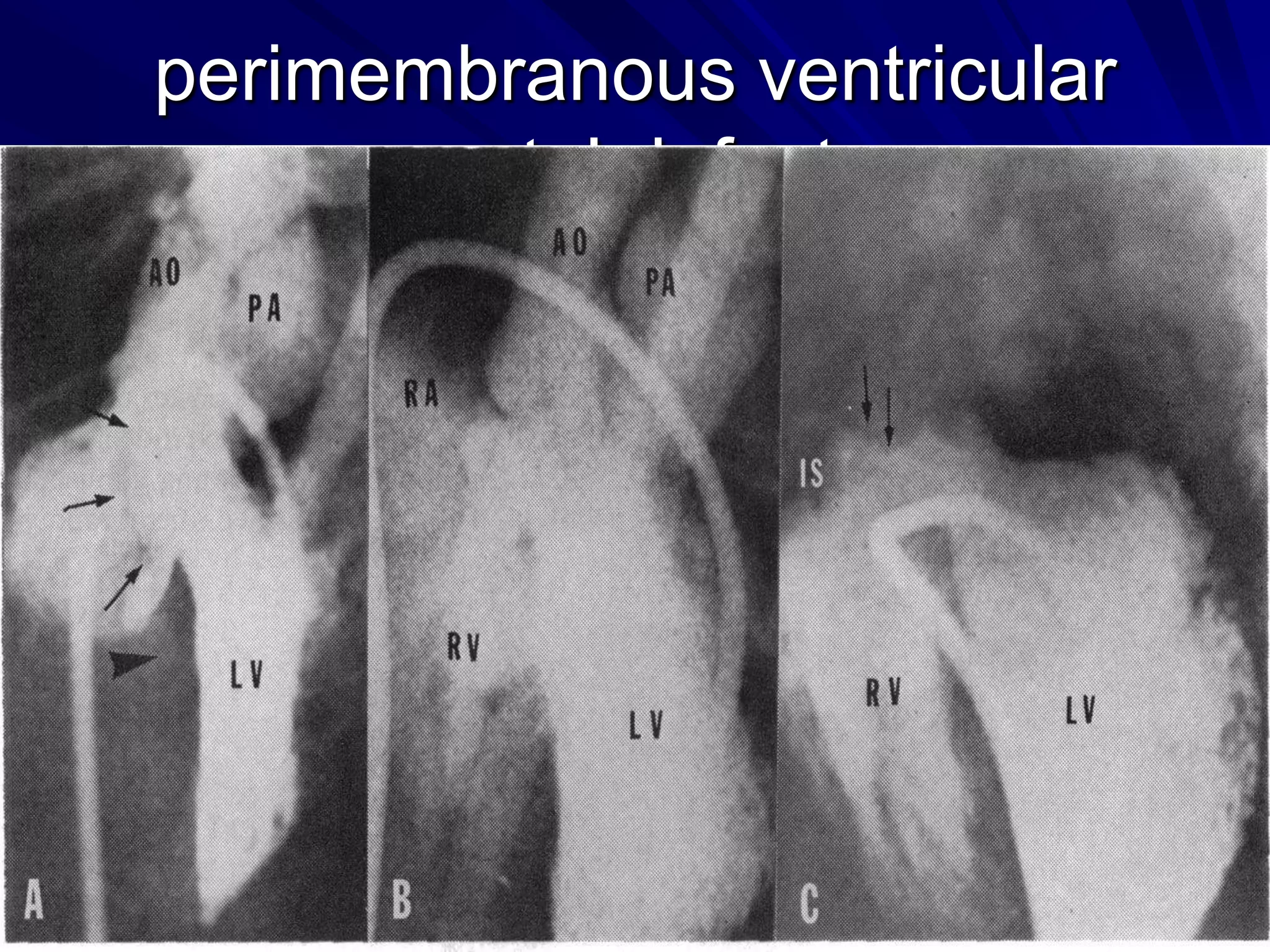
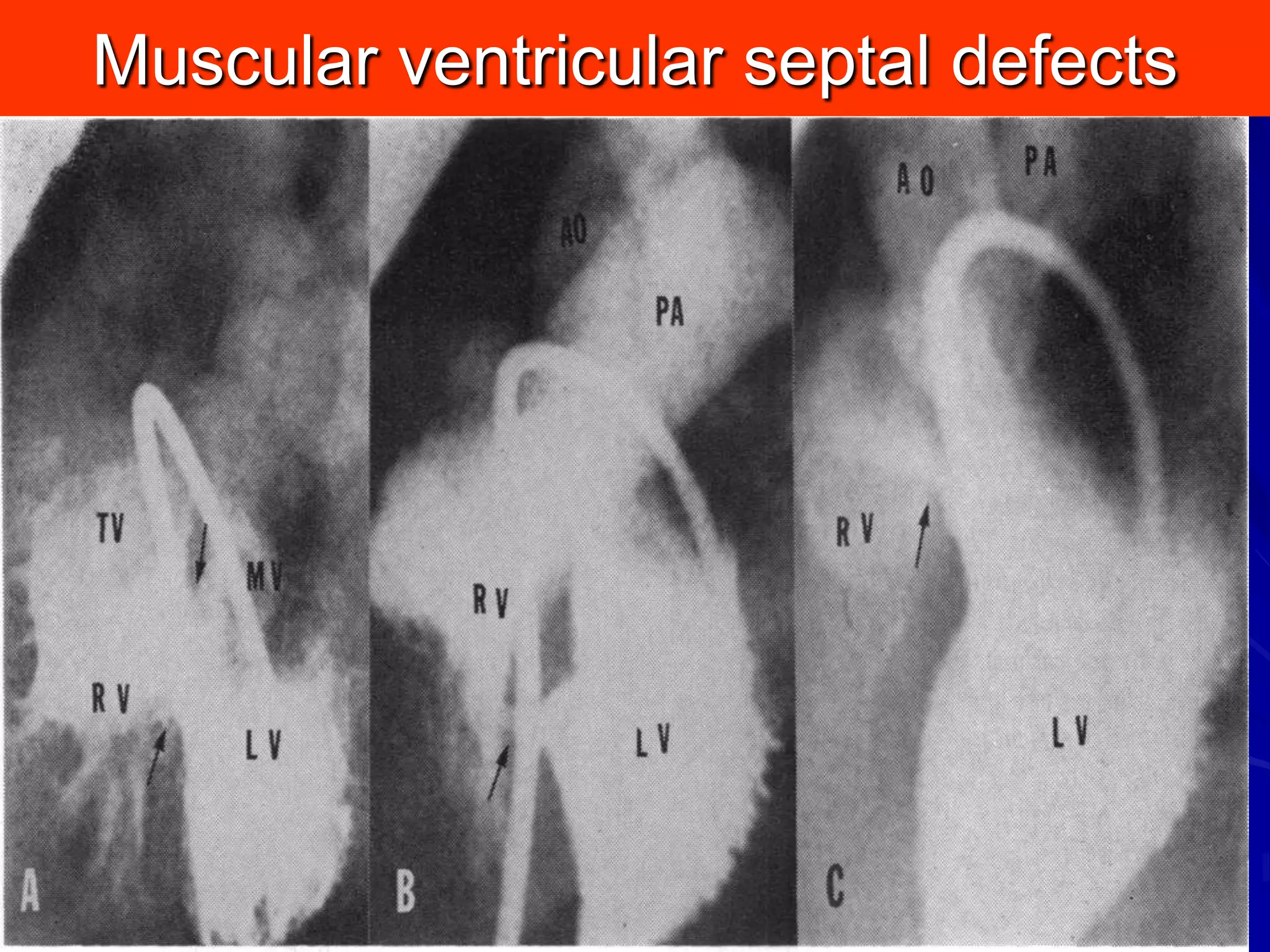
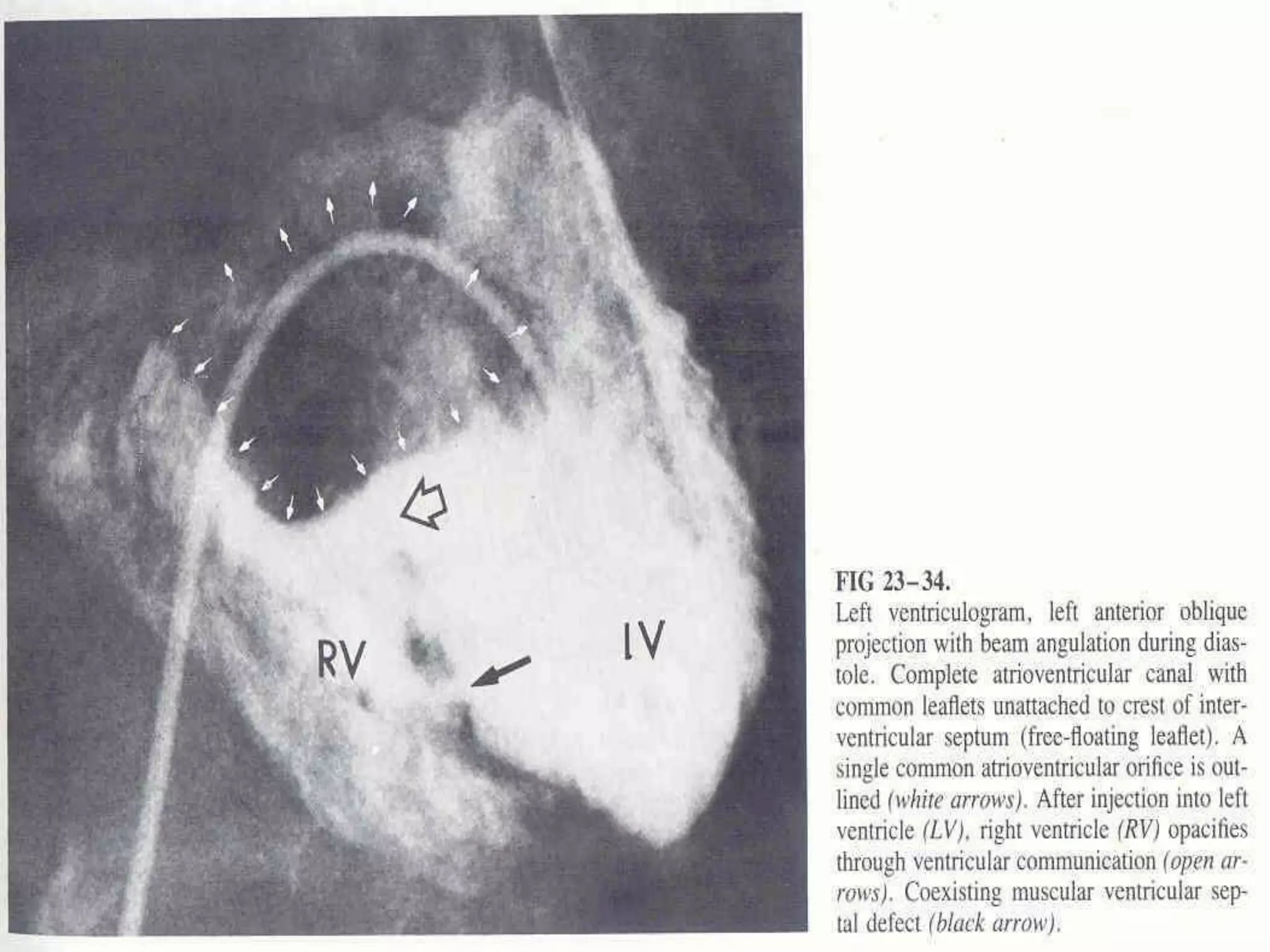
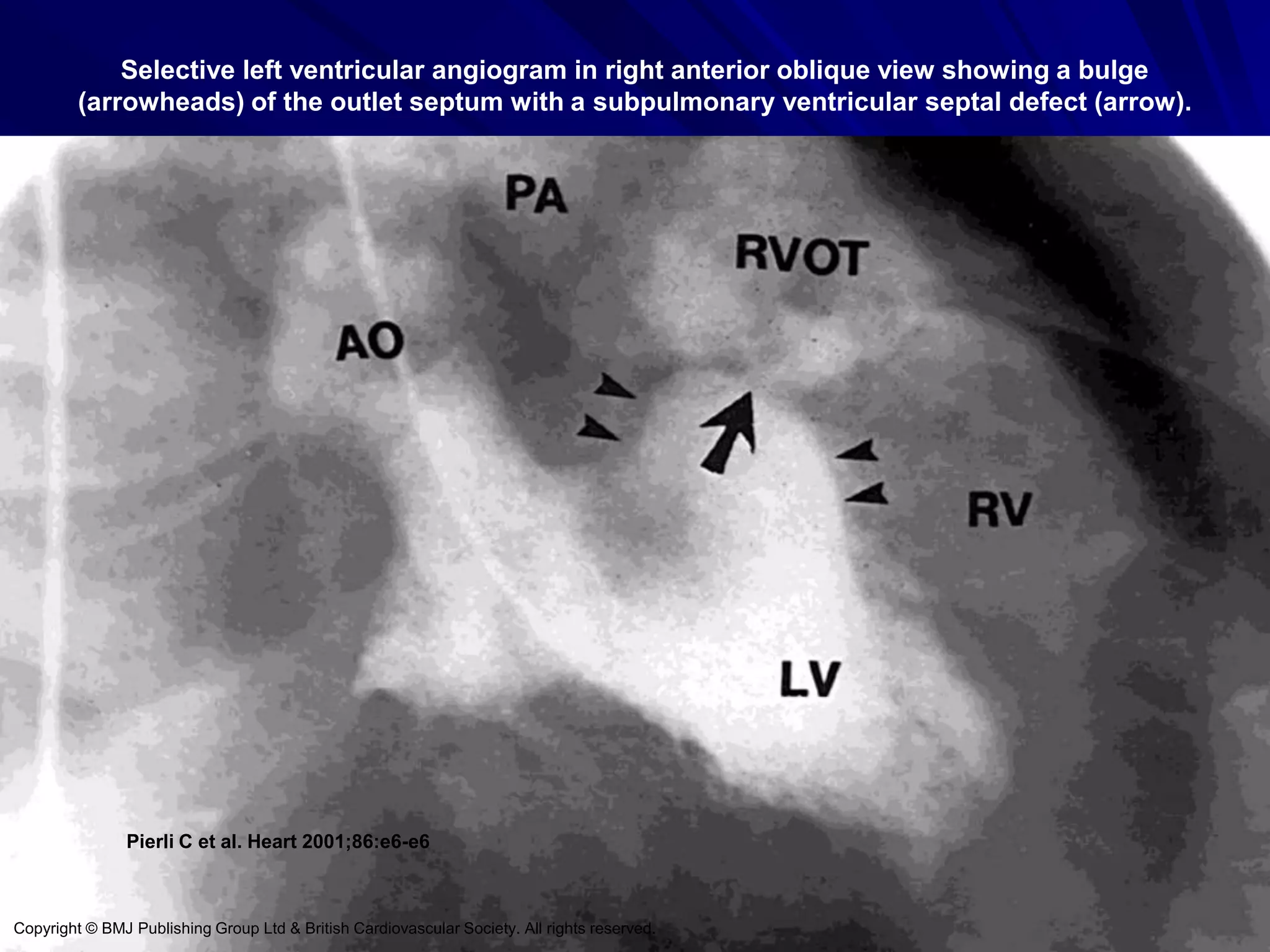
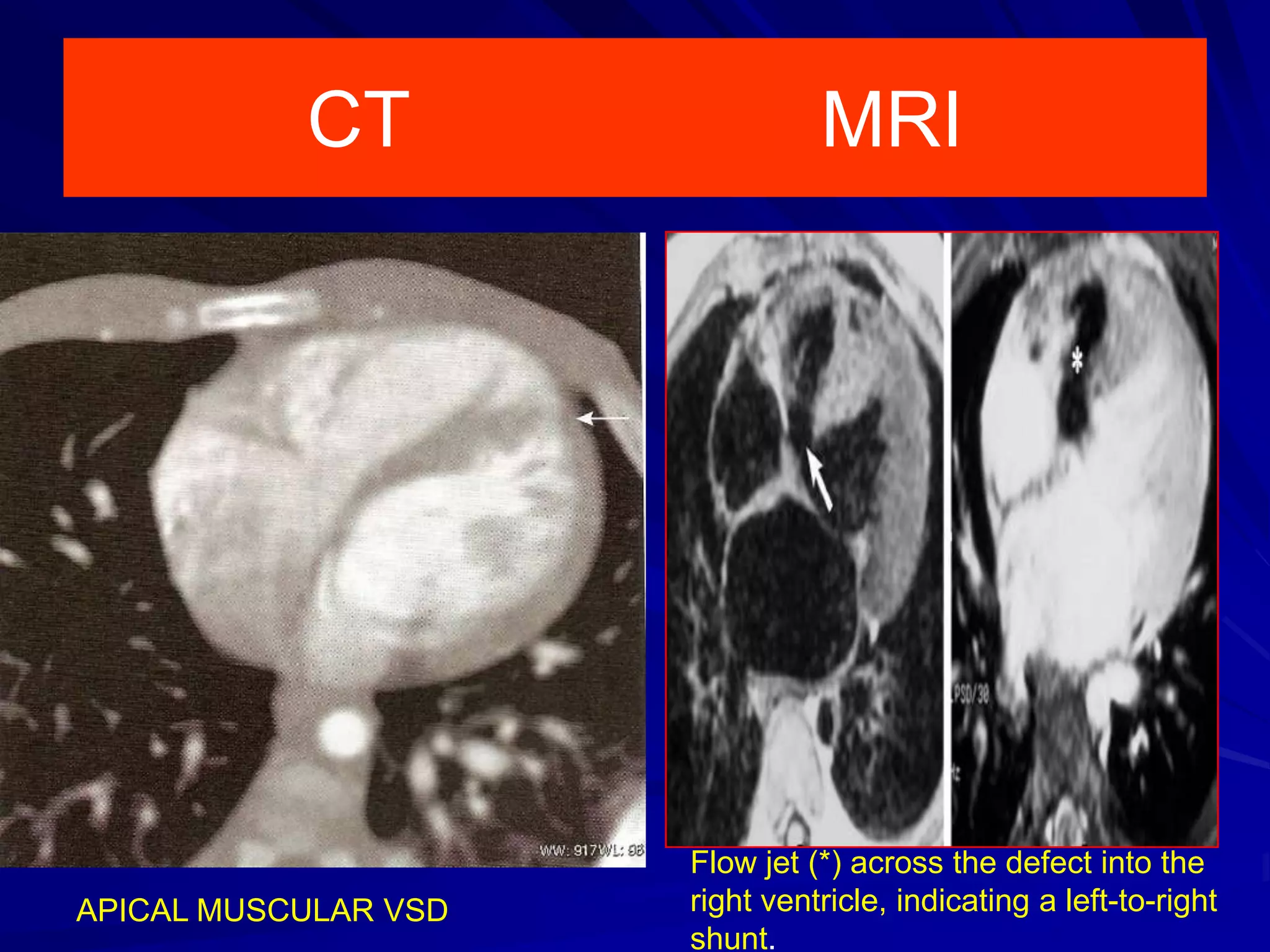
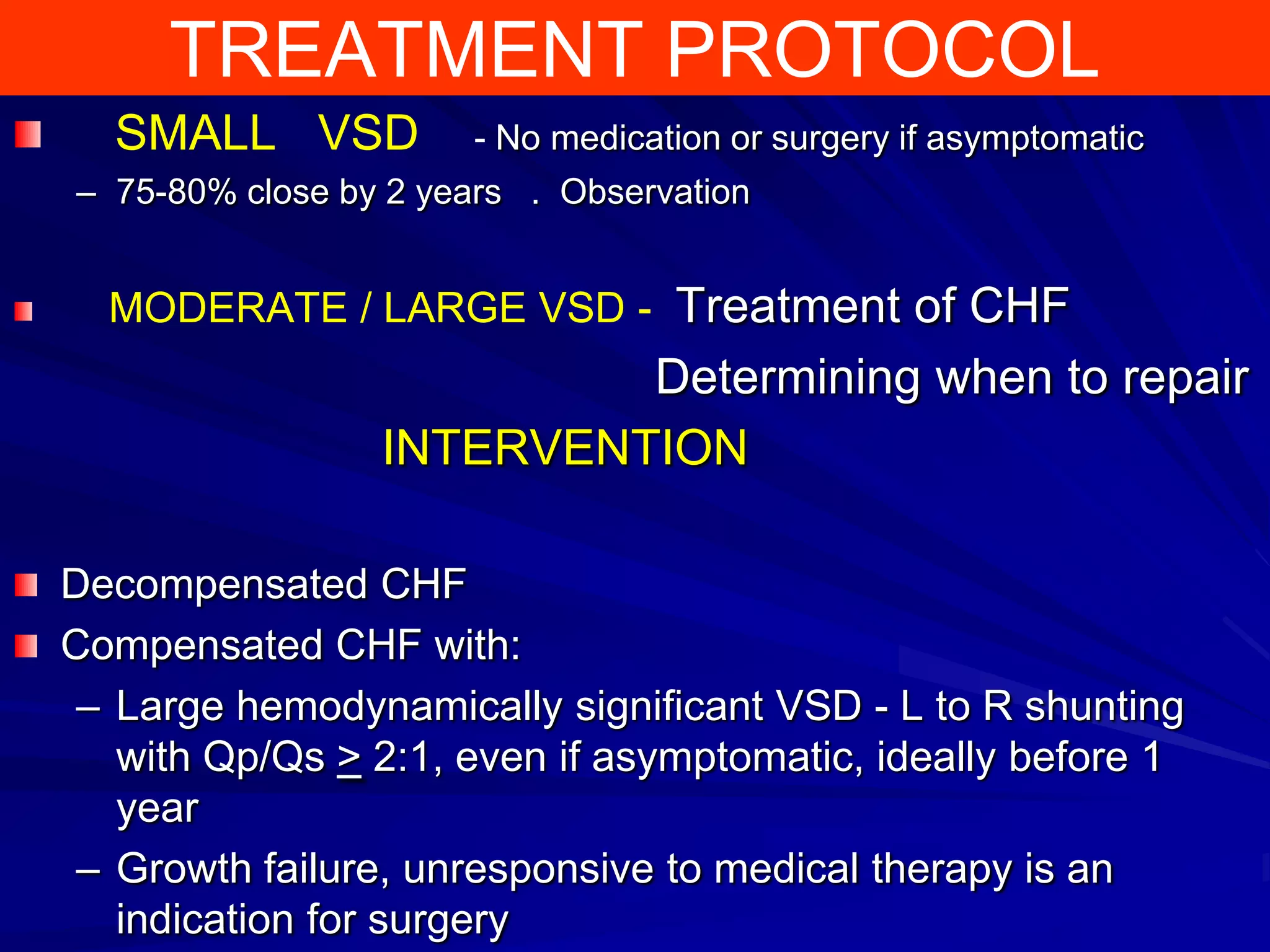
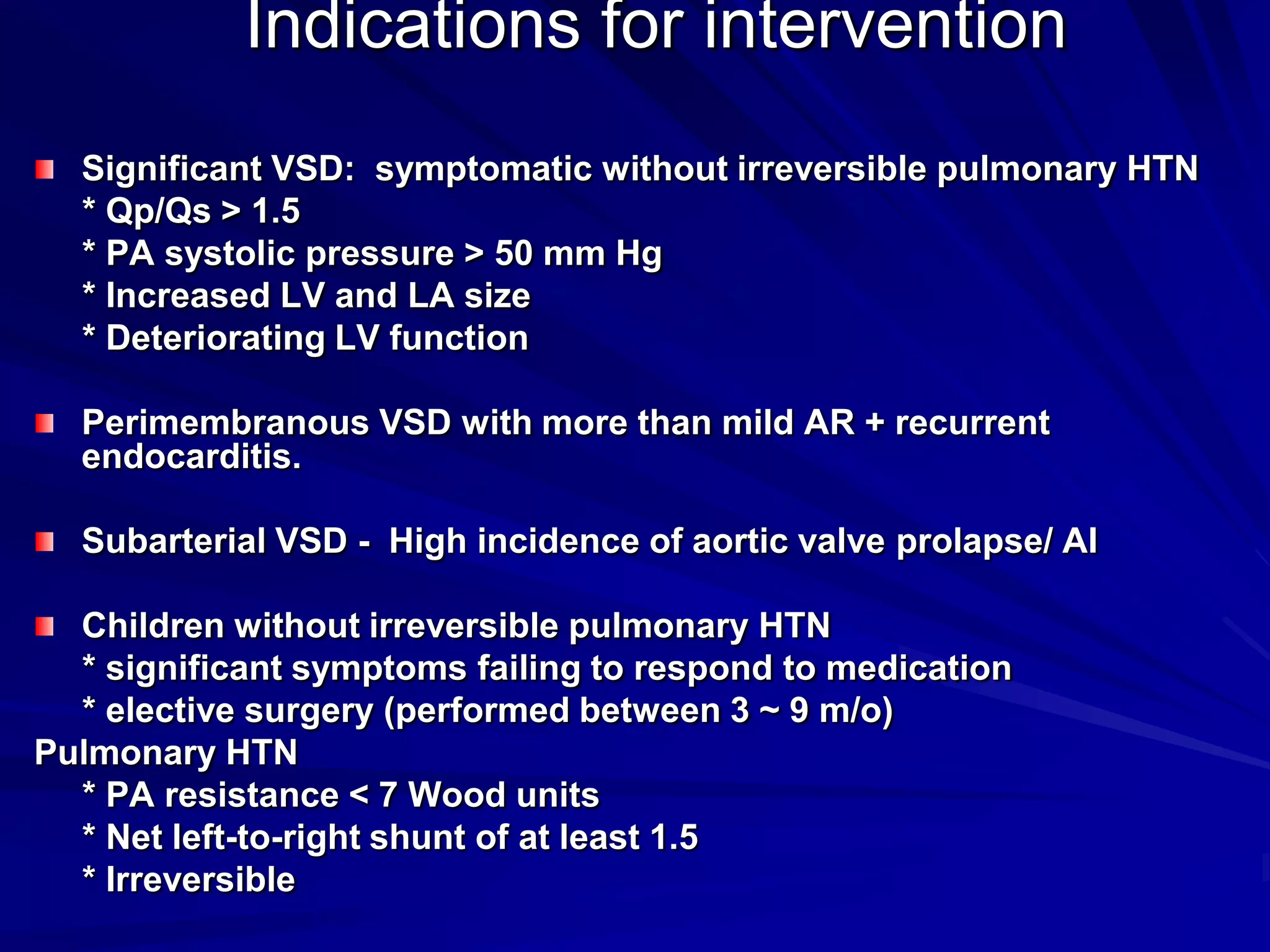
This document provides information on ventricular septal defects (VSDs), including their history, embryology, classification, pathophysiology, clinical features, and natural history. Some key points:
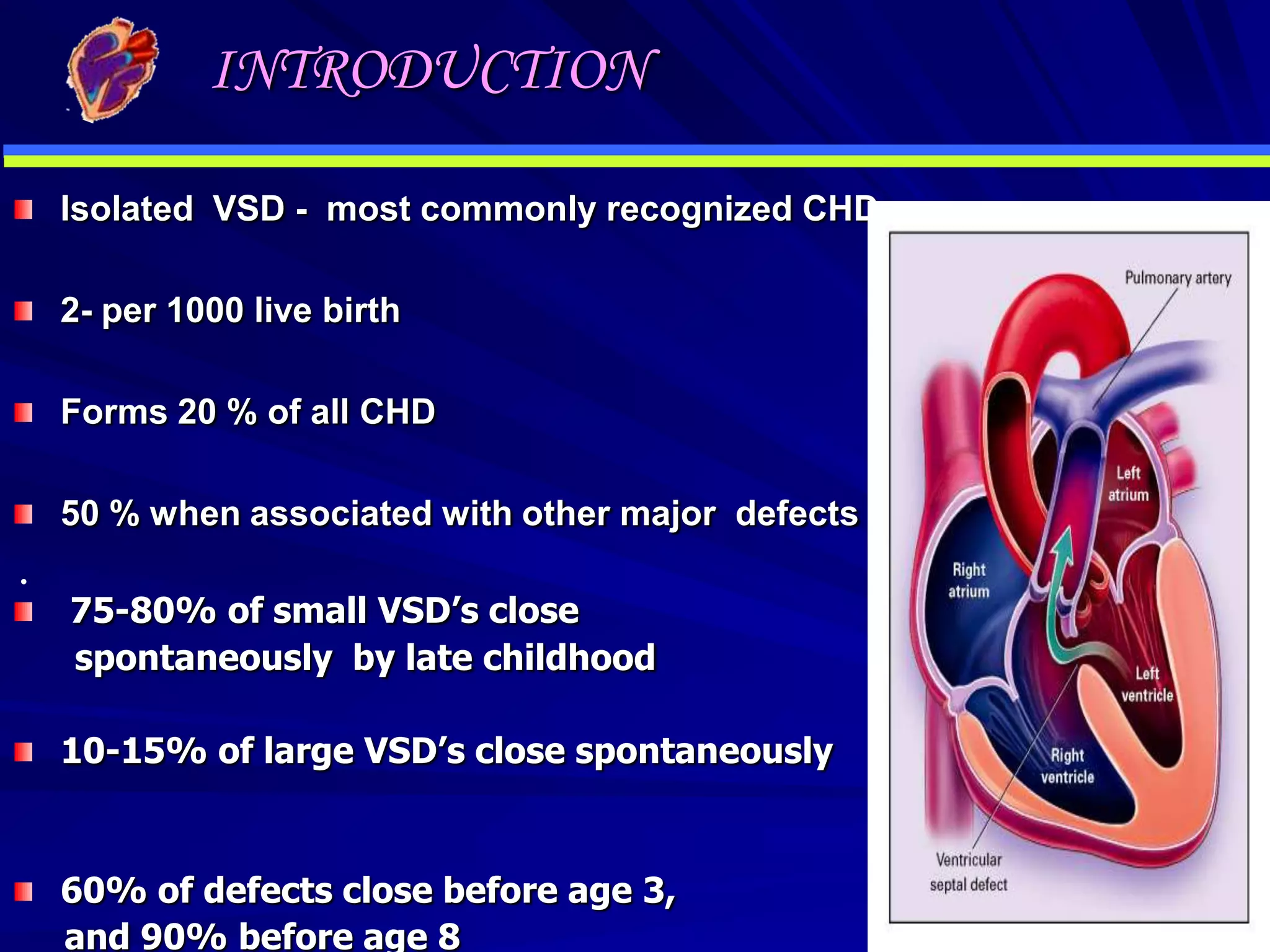
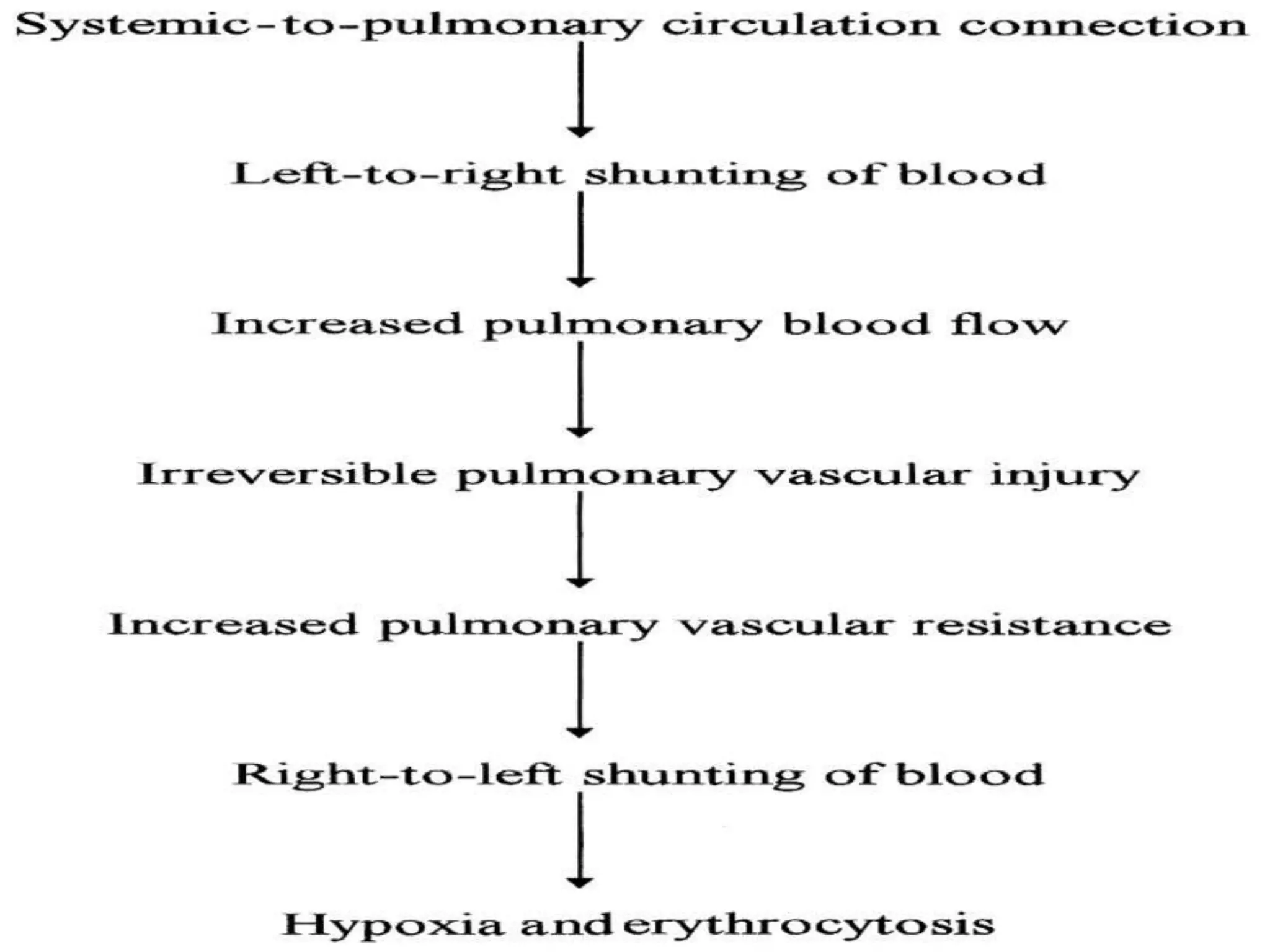
- VSDs are one of the most common congenital heart defects, occurring in around 2 per 1000 live births. They involve an abnormal opening in the wall separating the left and right ventricles.
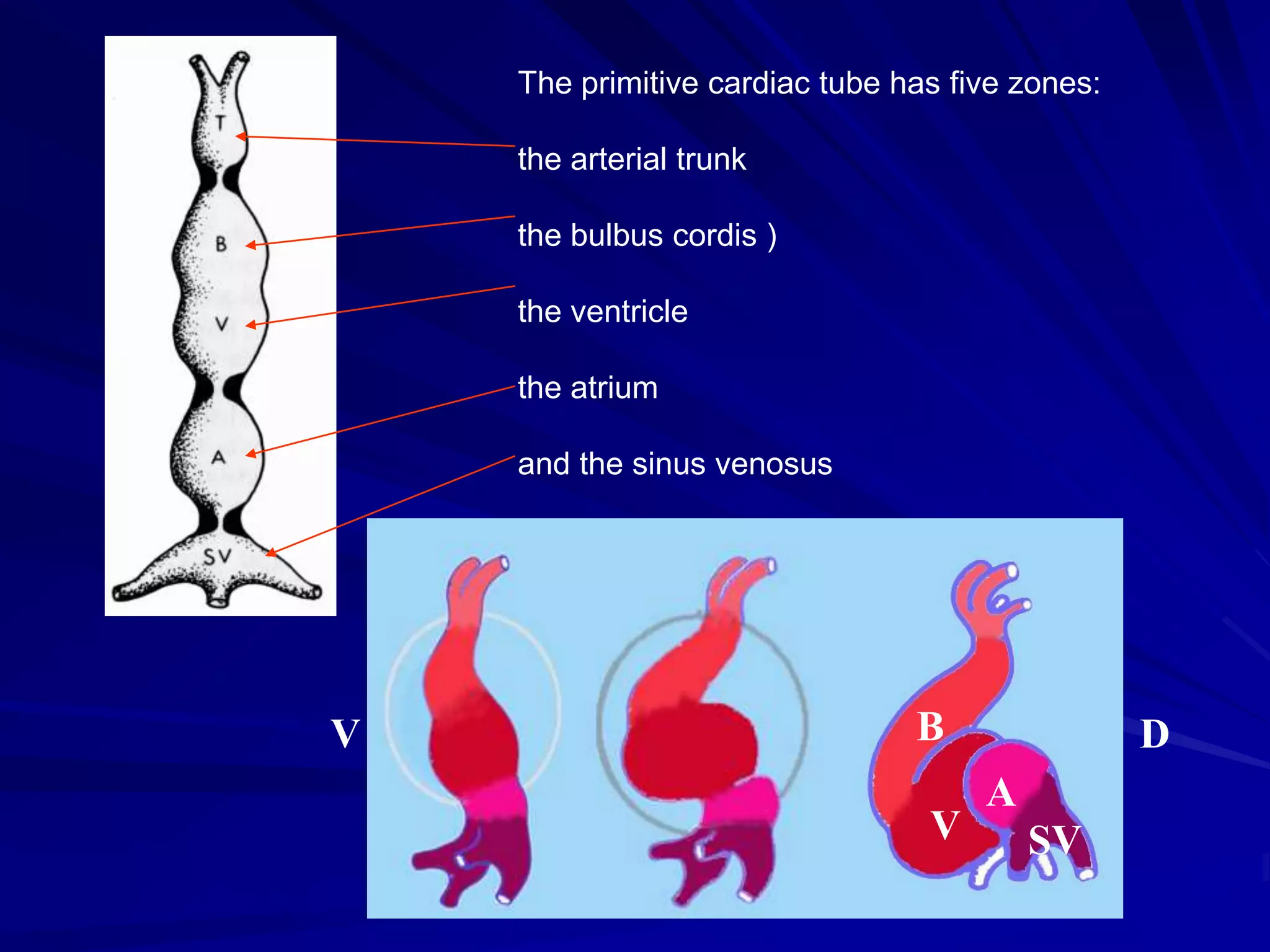
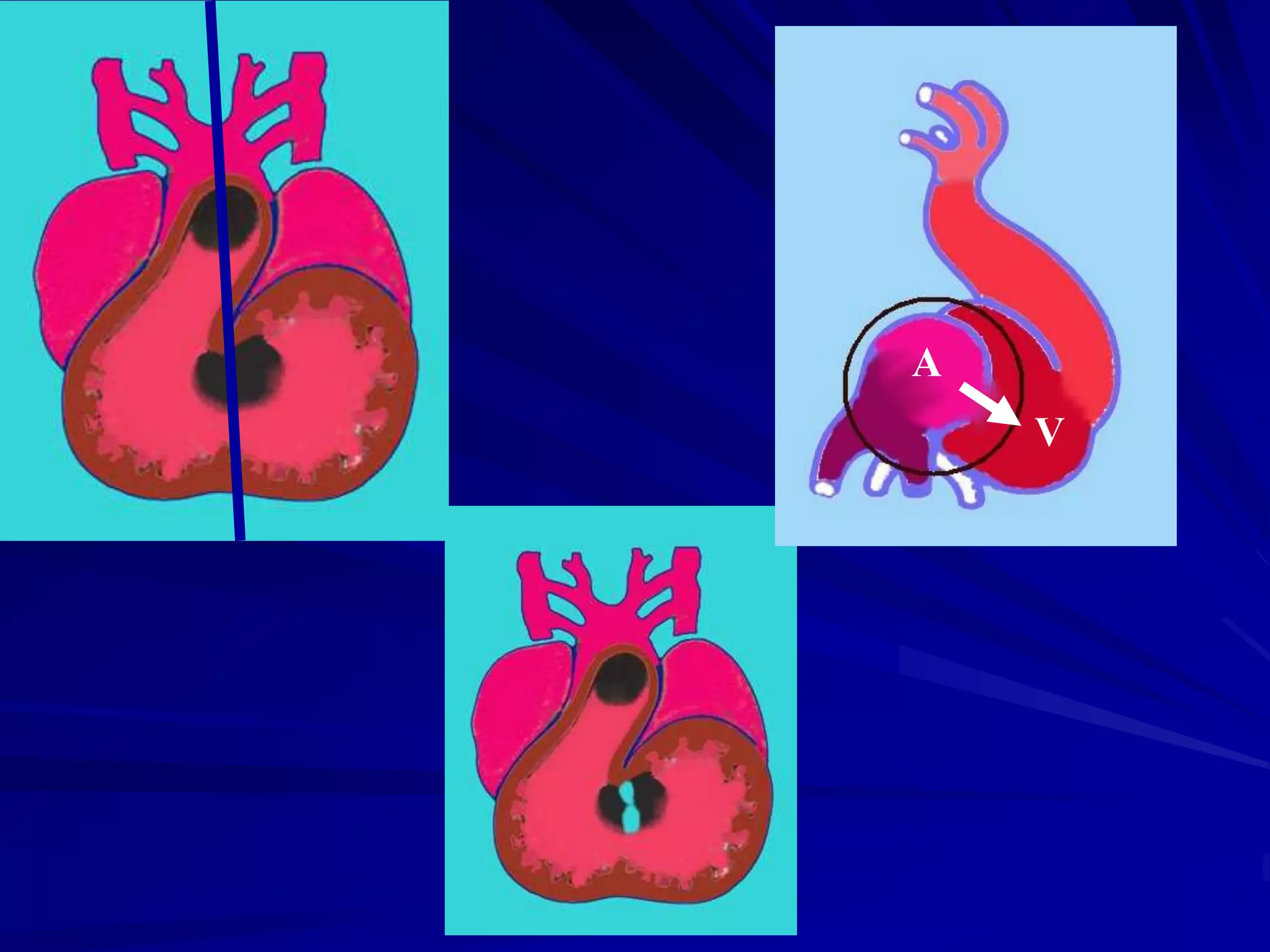
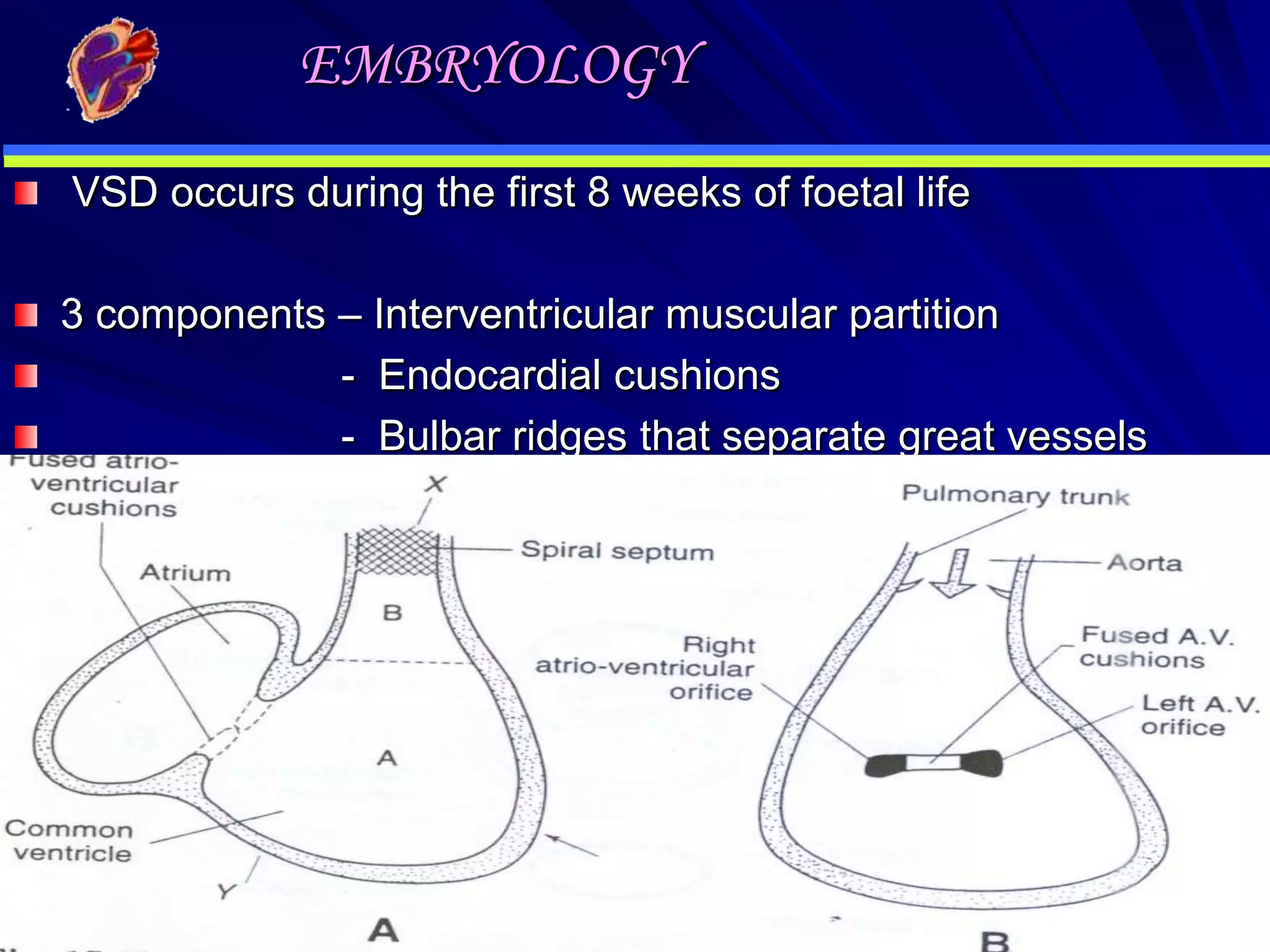
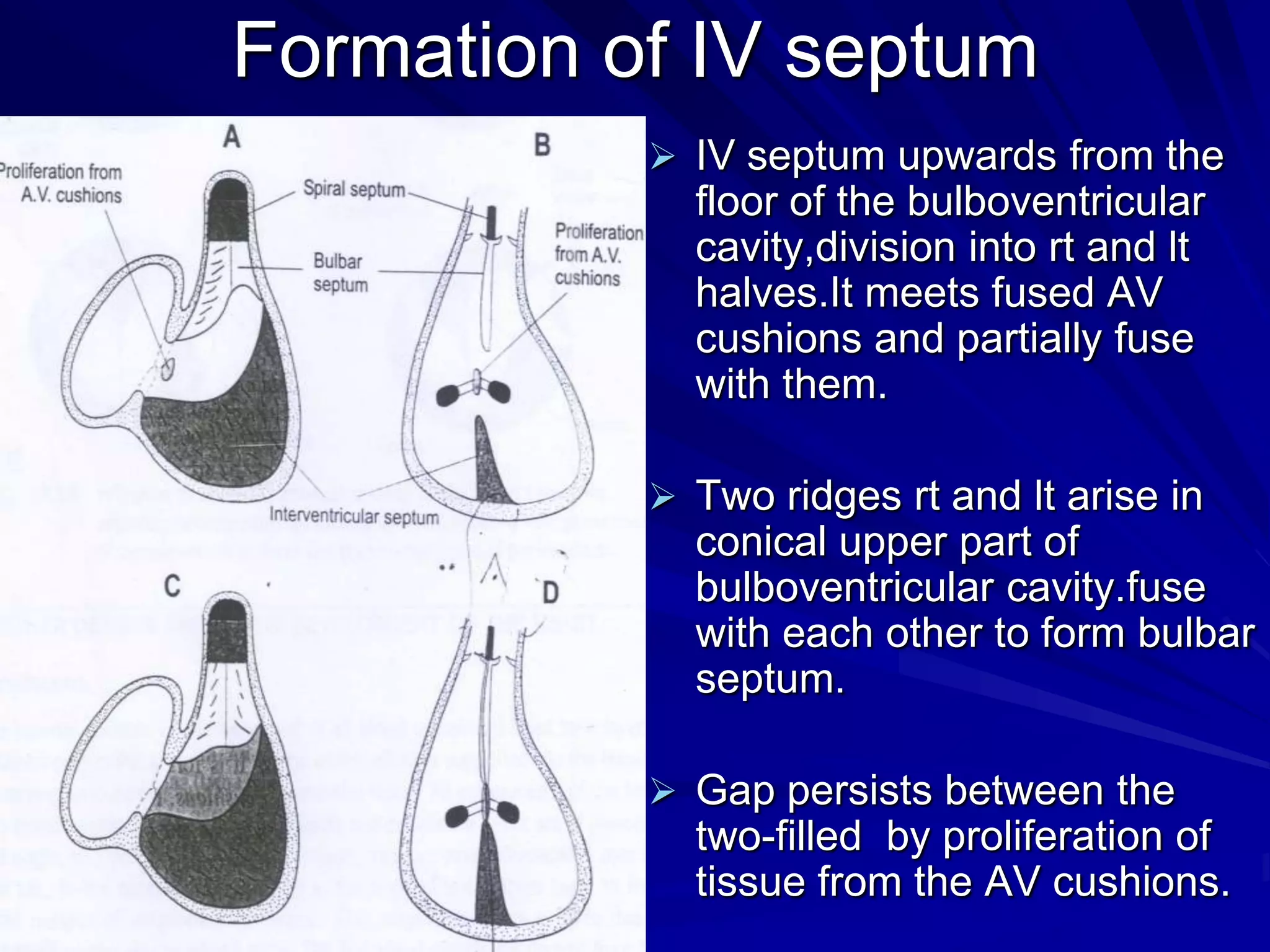
- Their formation occurs during the first 8 weeks of fetal development. Errors in the formation and fusion of the endocardial cushions and bulbar ridges can result in VSDs.
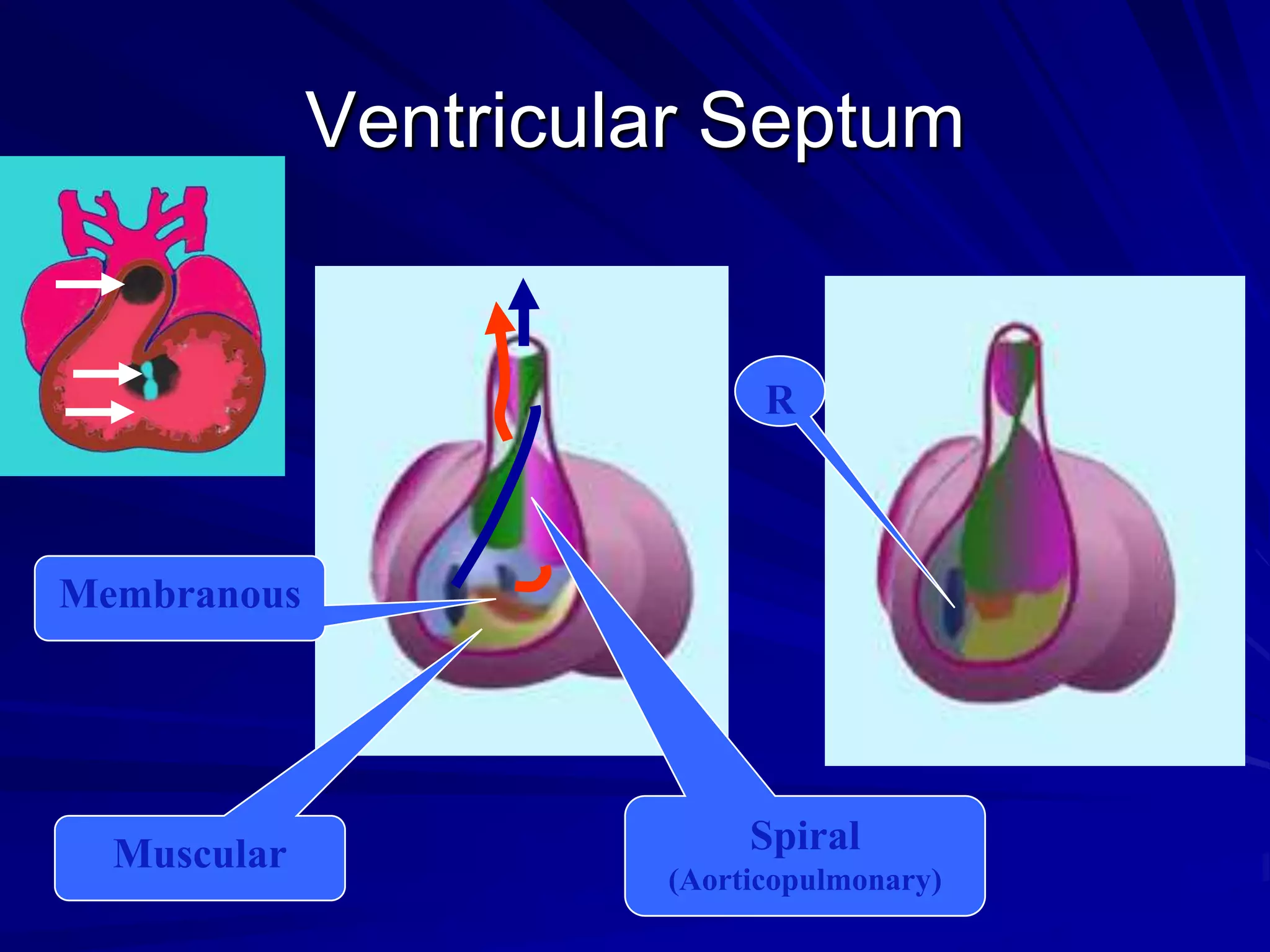
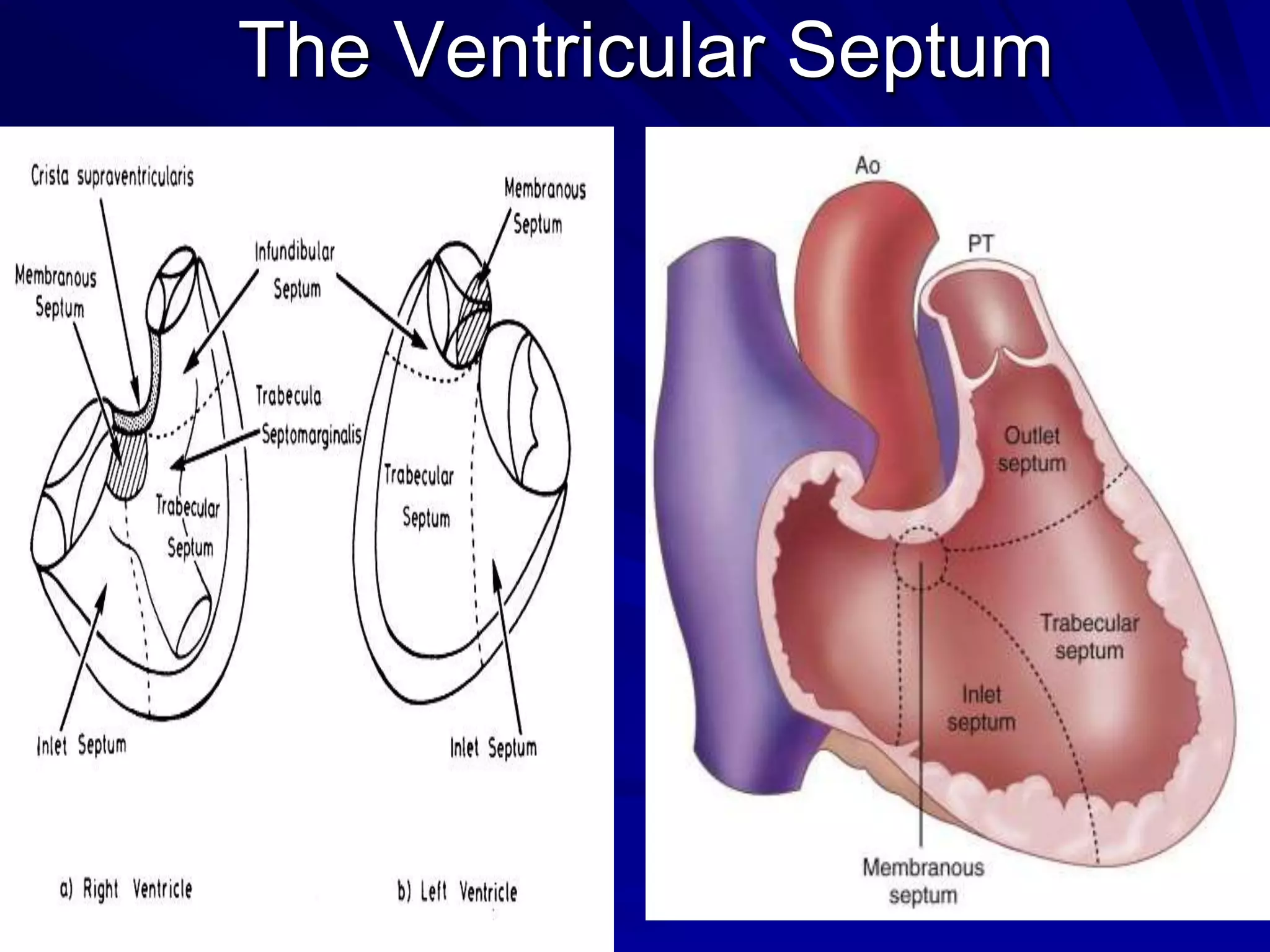
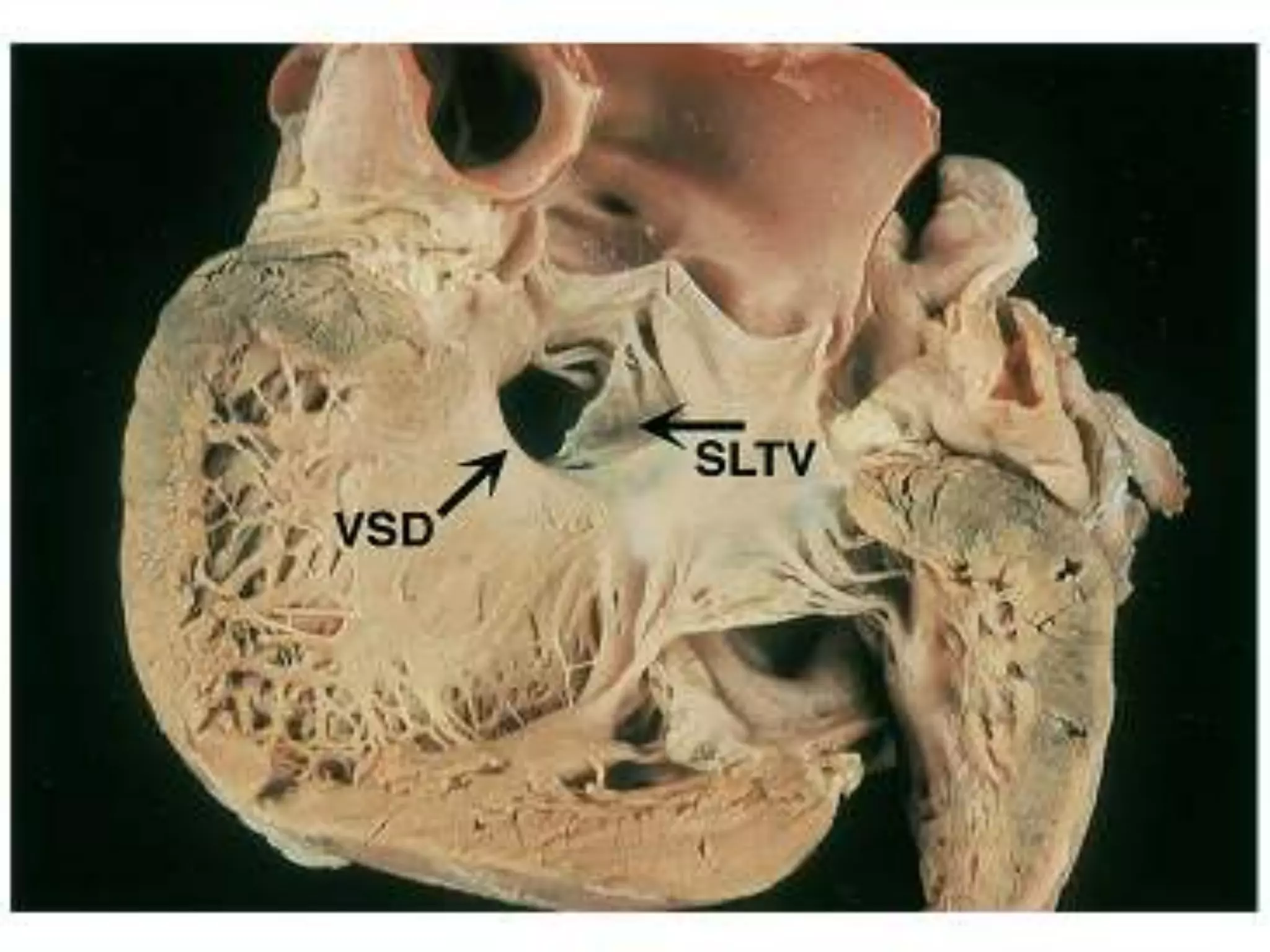
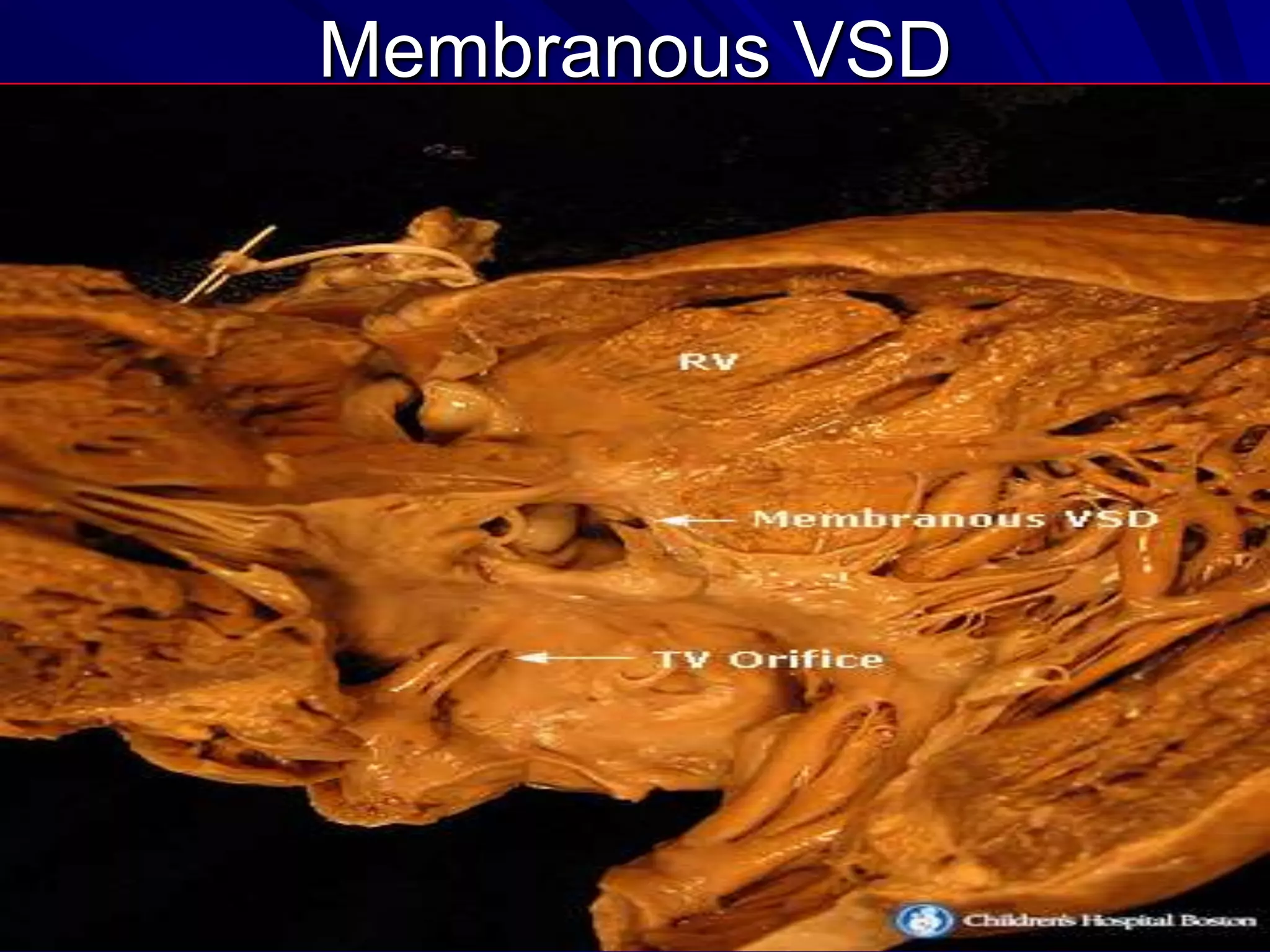
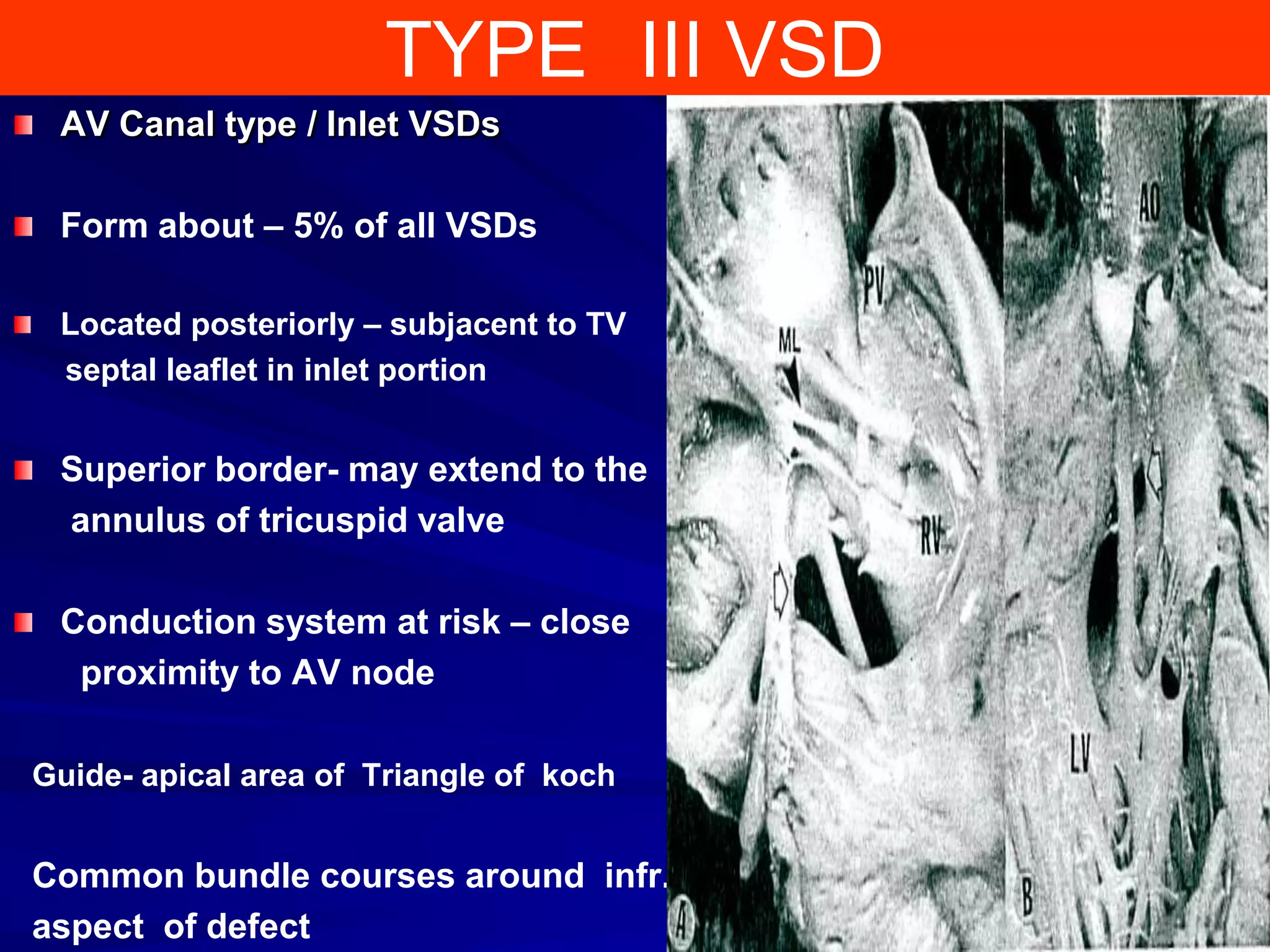
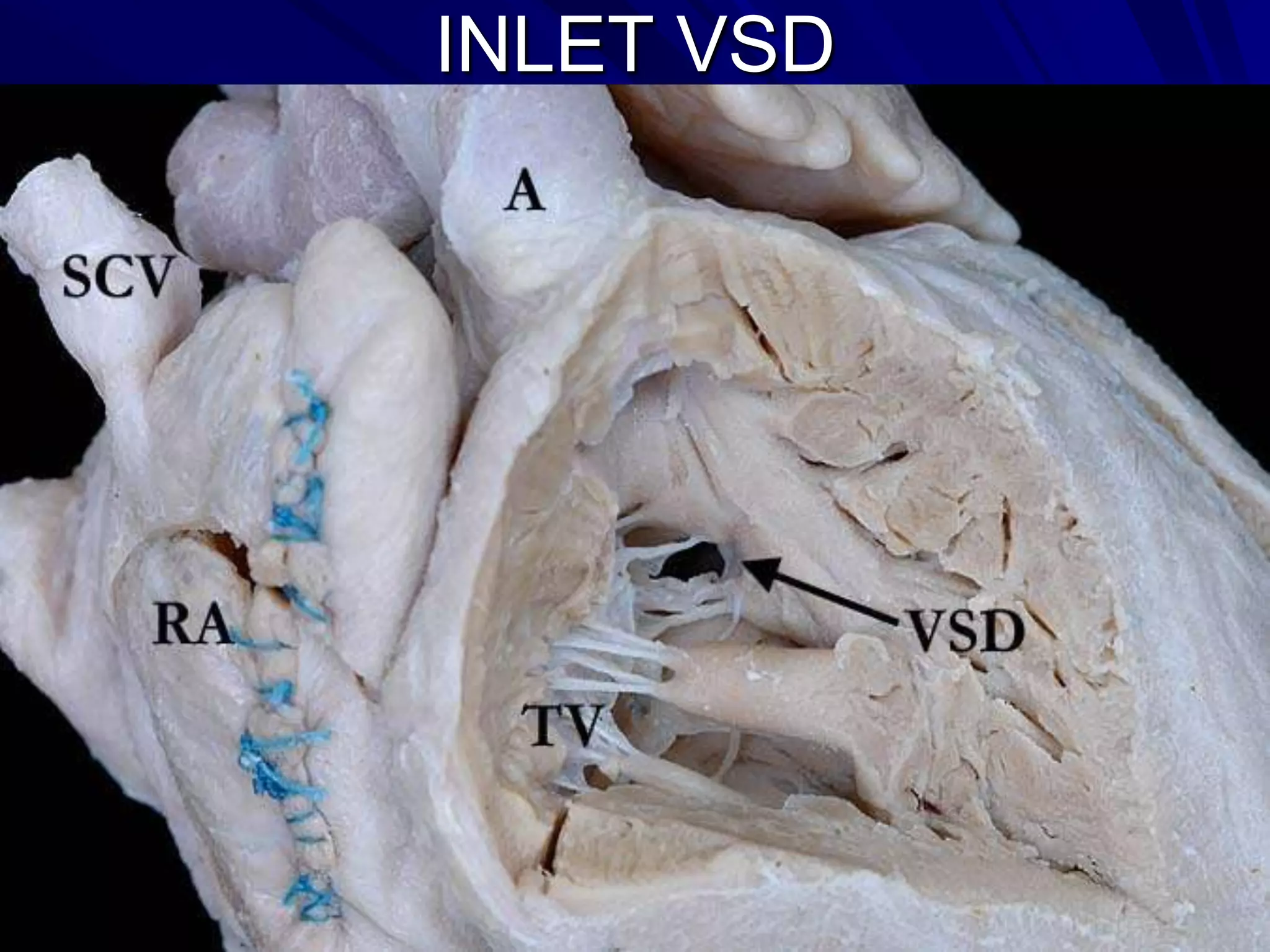
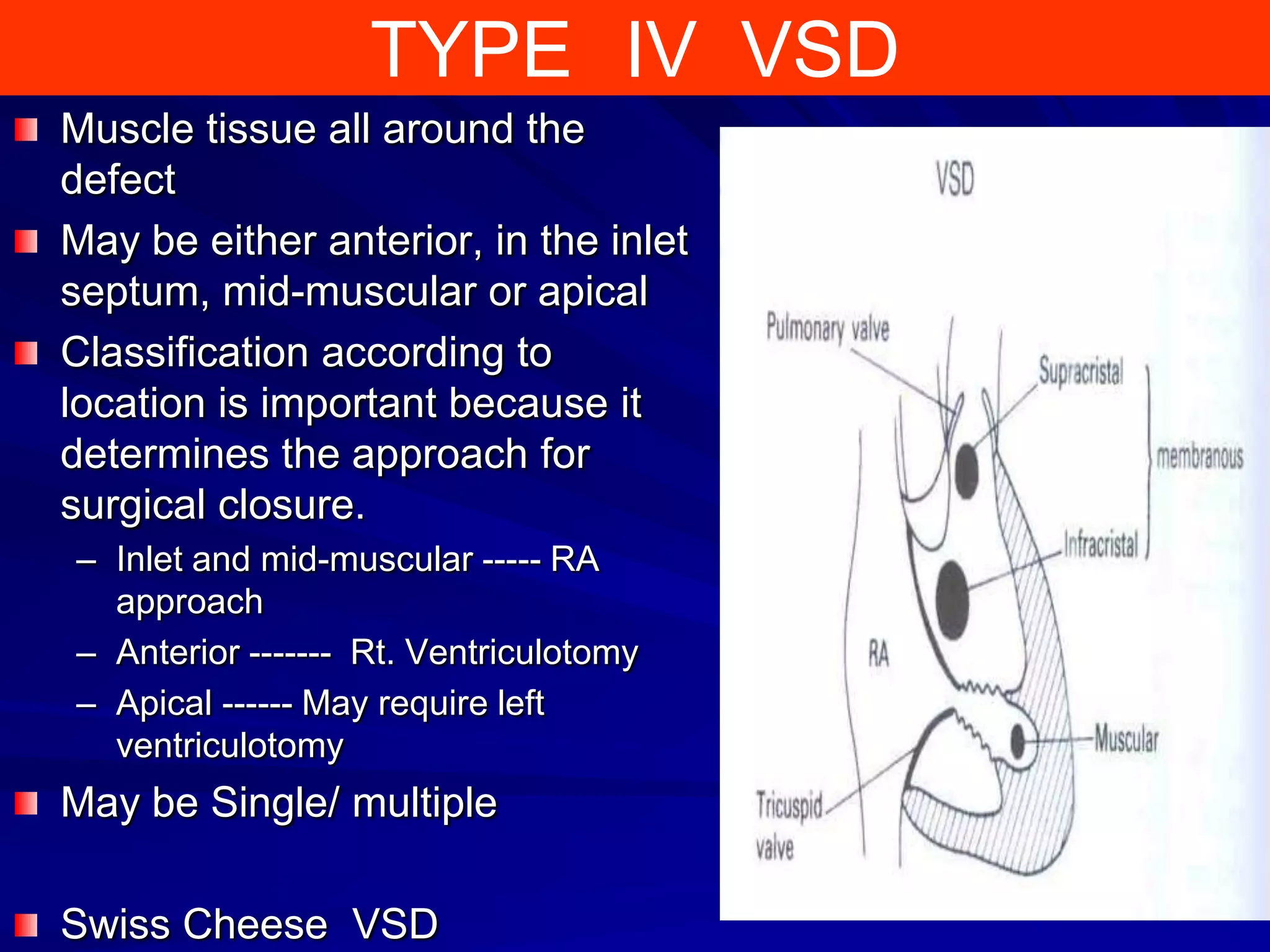
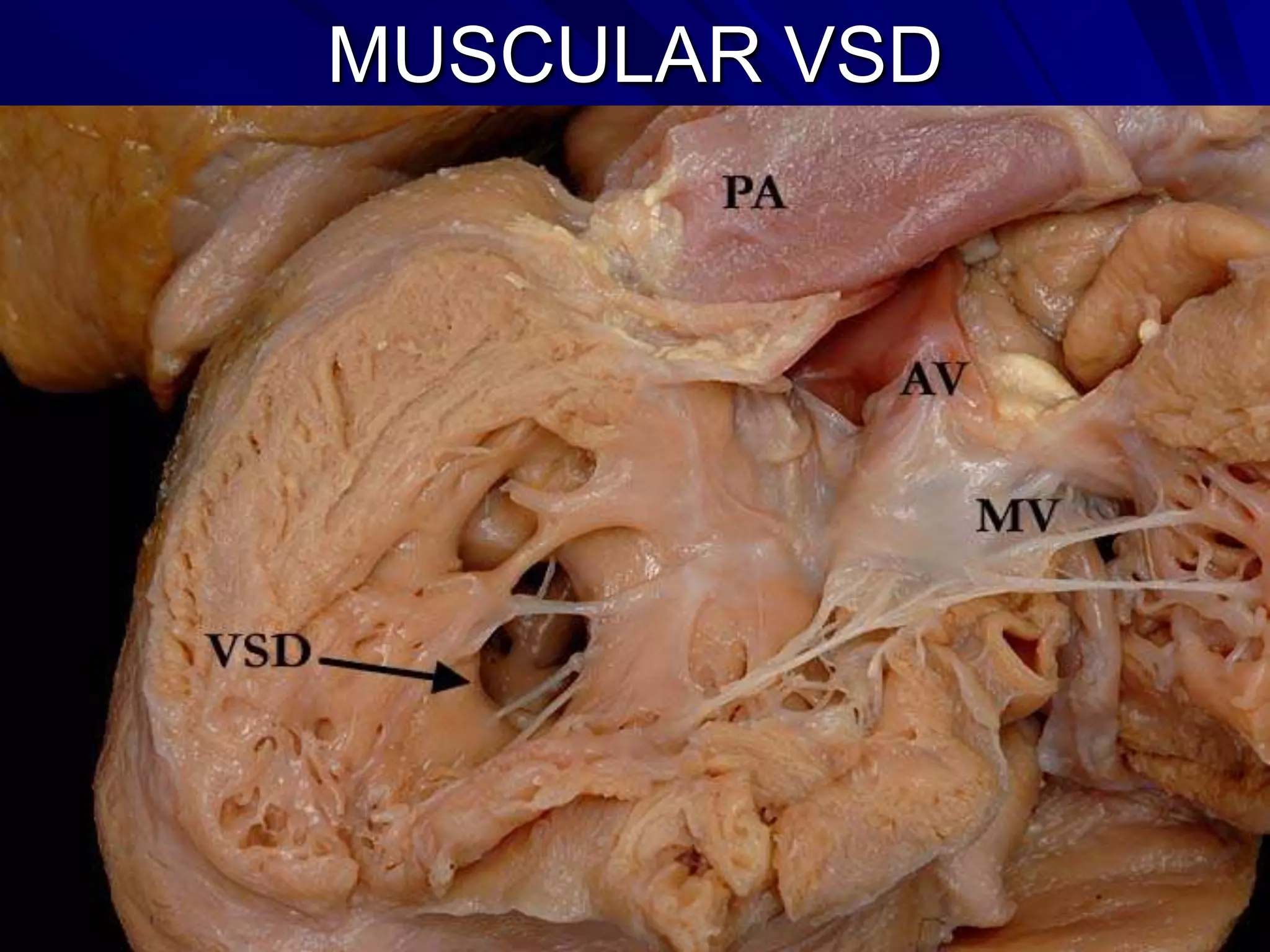
- VSDs are classified based on their location, with the main types being perimembranous, muscular,