This document discusses preformulation studies, which focus on the physical and chemical properties of a new drug compound and how those properties could impact drug performance and dosage form development. The goals of preformulation studies are to establish the physicochemical parameters, kinetics, stability, and compatibility of a new drug compound alone and when combined with excipients. Key physicochemical properties investigated include particle size, shape, crystallinity, solubility, hygroscopicity, and stability. Understanding these properties helps with rational dosage form design and evaluation of product efficacy and stability.



![I] Bulk characterization
1) Particle size
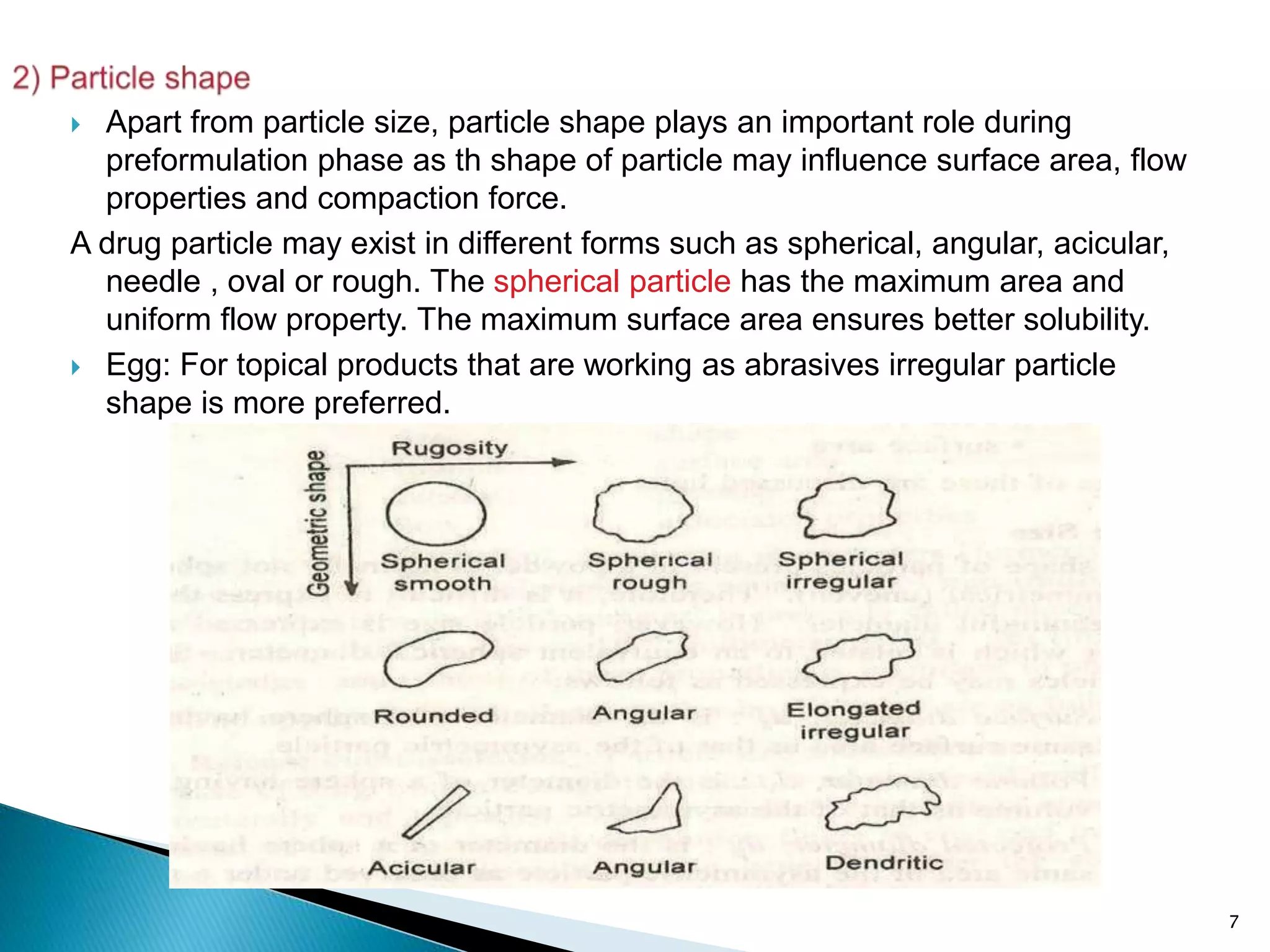
2) Particle shape
3) Crystallinity & polymorphism
4)Flow properties
5) Hygroscpicity
II] Solubility profile
i) pKa ii) pH iii) partition coefficient
III] Stability analysis
1) Particle size
Particle size is inversely proportional to the surface area. Properties such as
rate of absorption, dissolution, content uniformity and stability are
dependent to varying degrees on particle size, distribution and
interactions of solid surfaces.
Light microscopic method, Coulter counter method, HIAC counter are
methods for characterizing the size distribution of the compound
Sieve method for particle size ~ 100 microns.
4](https://image.slidesharecdn.com/preformulationstudies3-200829043054/75/Preformulation-studies-4-2048.jpg)





















![ The solubility of weakly acidic and weakly basic drug as function of
pH can be predicted with the help of equation,
Where, S = solubility at given pH.
So = intrinsic solubility of neutral form.
K1 = dissociation constant for the weak acid.
K2 = dissociation constant for weak base.
The intrinsic solubility must be measured at two temperatures
a) 4˚C- To ensure physical and chemical stability
b) 37˚C- To support biopharmaceutical evaluation
27
S = So {1 + (K1/ [H+])} For weak acid
S = So {1+ ([H+]/ K2)} For weak base](https://image.slidesharecdn.com/preformulationstudies3-200829043054/75/Preformulation-studies-27-2048.jpg)

![ The Henderson-Hasselbalch equation provides an estimate of the
ionized and un-ionized drug concentration at a particular pH
For acidic compounds:
pH = pKa + log ([ionized drug]/[un-ionized drug])
For basic compounds:
pH = pKa + log ([un-ionized drug]/[ionized drug])
The various methods for determination
of pKa are:
a) Potentiometric method
b) Spectrophotometric method
c) Solubility method
d) Conductivity method
29](https://image.slidesharecdn.com/preformulationstudies3-200829043054/75/Preformulation-studies-29-2048.jpg)





















![POLYMERIZATION
Polymers are high molecular weight substances, fashioned by
aggregation of smaller molecules called monomers.
It is a continuous reaction between molecules.
More than one monomer reacts to form a polymer.
E.g. Darkening of glucose solution is attributed to polymerization of
breakdown product [5- (hydroxyl methyl) furfural].
E.g. Shellac on aging undergoes polymerization & hence prolongs
disintegration time & dissolution time.
51](https://image.slidesharecdn.com/preformulationstudies3-200829043054/75/Preformulation-studies-51-2048.jpg)