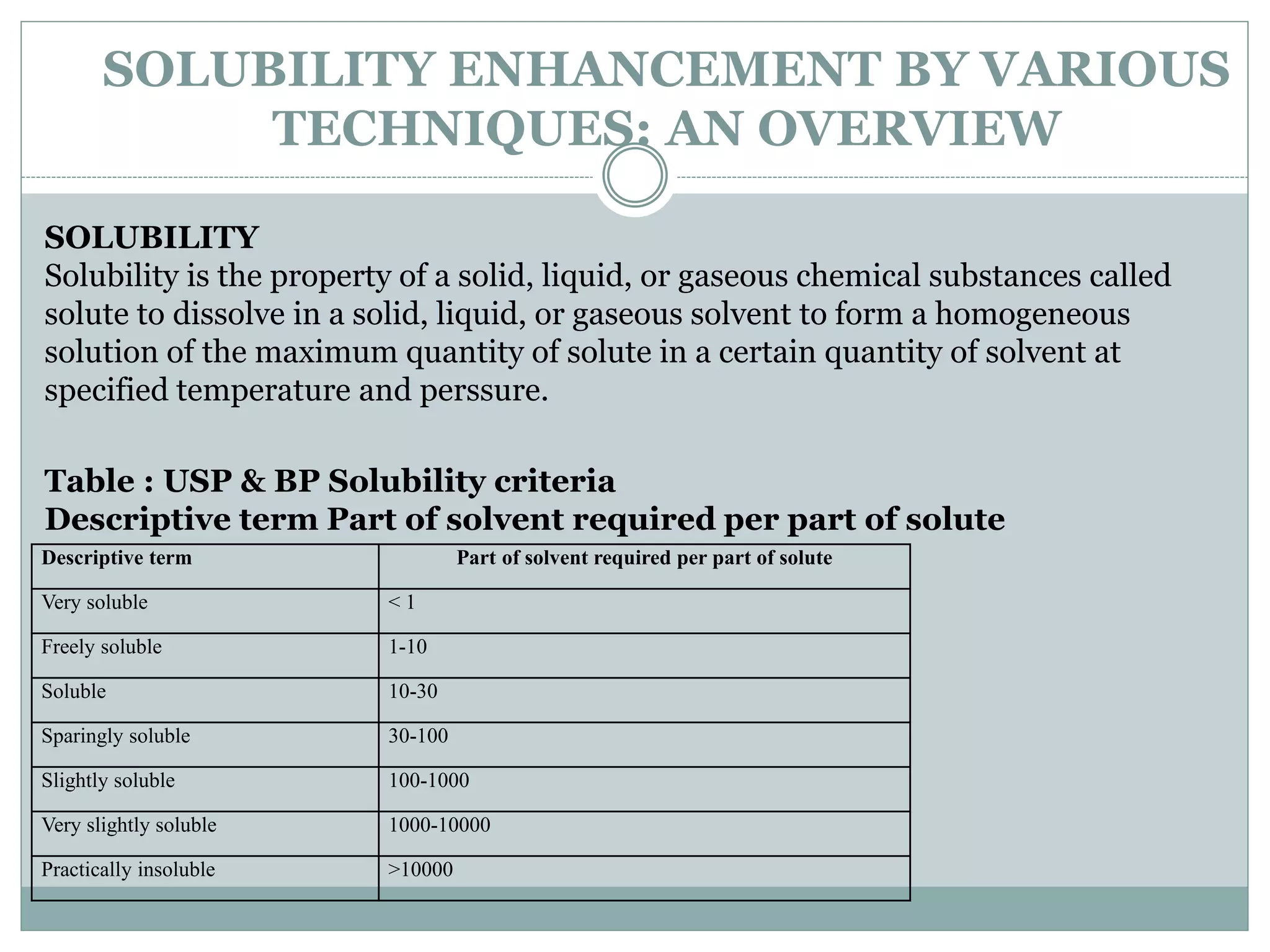
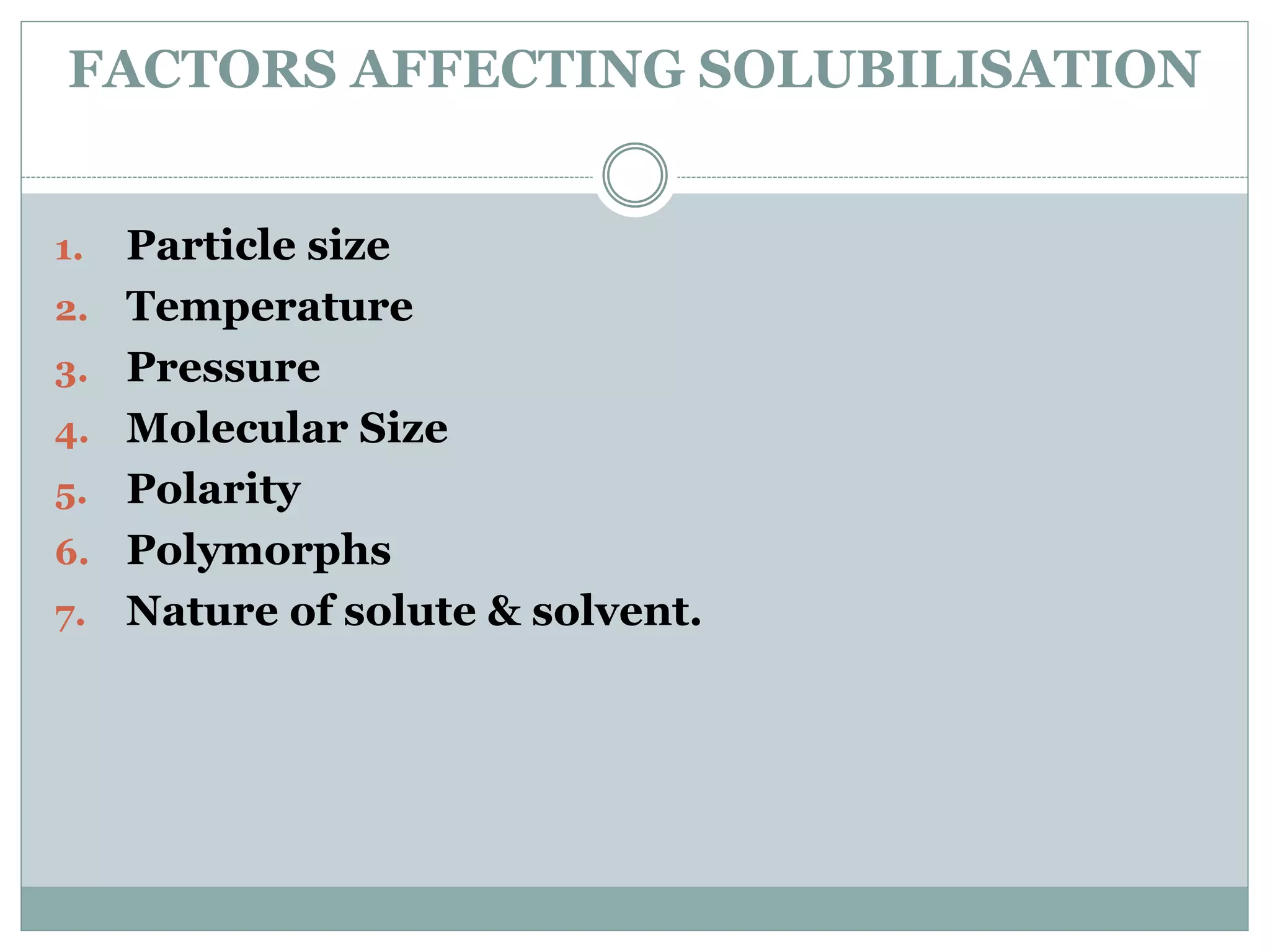
This document provides an overview of solubility enhancement and cosolvency. It begins with definitions of solubility enhancement and cosolvency as using water-miscible solvents to increase the solubility of weak electrolytes and nonpolar molecules. Common cosolvents like ethanol, propylene glycol, and polyethylene glycol are discussed. The mechanisms of how cosolvents increase solubility by changing dielectric constant and promoting hydrogen bonding are explained. Several methods for solubility enhancement are reviewed, including particle size reduction, use of surfactants, solid dispersions, and pH adjustment. The document concludes that various techniques can be combined to improve solubility of poorly soluble drugs based on their properties, with the goal of improving bioavailability



![REVIEW LITERATURE
1. S.K. dash et al(2012) [12] : solubility enhancement of poorly poorly water
drugs. Crit Rev Ther Drug Carrier Syst. 2002; 19:553-585.
2. Chellos N.et al(2012)[13]: “cosolvency and soluility enhancement”
Pharmatech 2003, 160-166. They had developed simultaneous determination of
sitagliptin phospate monohydrate and metformin by ultra performance liquid
chromatographic (uplc)method. the chromographic sepreation was achieved on
aquity uplc BEH C8 100 X 2.1mm,1.7 m, colunm using a buffer consisting of 10
mM potassium dihydrogen phosphate and 2 nm hexane 1-sufonic acid sodiun,
salt(PH adjusted to 5.50 with diluted phosphoric acid) and acetonitrile as organic
solvent in a gradient program. The flow rate was 0.2 ml min-1 and the detection
wavelength was 210nm.the limit of detection (LOD) was 0.2 and 0.06 g ml-
1,respectively.the limit of quantification (LOD)was 0.7 and 0.2g ml-
1,respectively.this method was validated with respect to linearity,
accuracy,precision ,specificity,and robustnes soluble drugs . . Crit Rev Ther Drug
Carrier Syst. 2002; 19:553-585.](https://image.slidesharecdn.com/04madhavi-180104112710/75/solubility-enhancement-and-cosolvency-by-madhavi-4-2048.jpg)