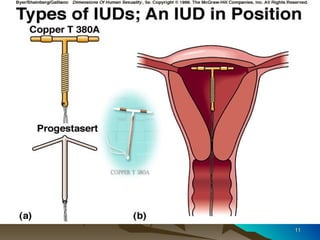
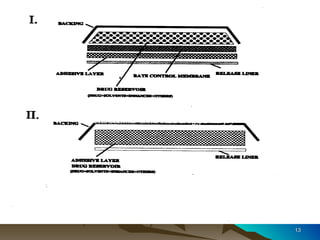
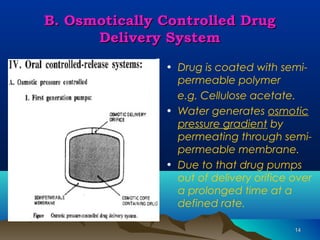
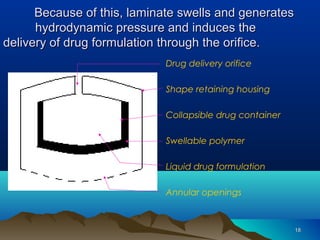
The document discusses different types of polymers used in pharmaceutical applications. Polymers are long chain molecules formed from linking smaller molecules called monomers. They can be homopolymers made of identical monomers or copolymers with two or more different monomers. The document then classifies polymers based on origin, biodegradability, reaction type, and interaction with water. It provides examples of controlled drug delivery systems that use polymers, including reservoir, matrix, biodegradable, osmotic, and swelling-controlled release systems.


![33
• Copolymer :
Polymers formed from two or
more different monomers are called as
copolymers.
- [A – B – A – B – A – B] –
• Homopolymer :
Polymers formed from bonding of
identical monomers are called as
homopolymers.
- [A – A – A – A – A] -](https://image.slidesharecdn.com/polymersciencefordrugdelivery-140930004323-phpapp02/85/Polymer-sciencefordrugdelivery-3-320.jpg)