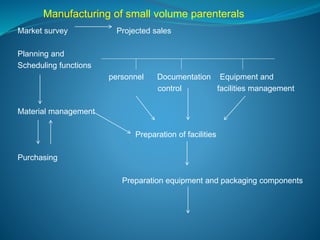
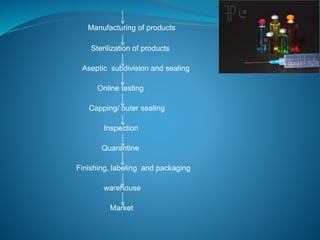
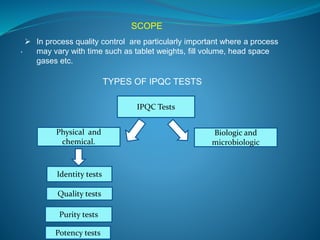
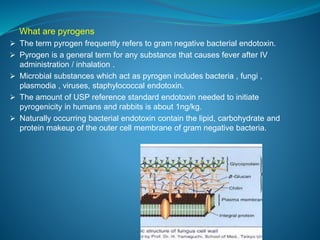
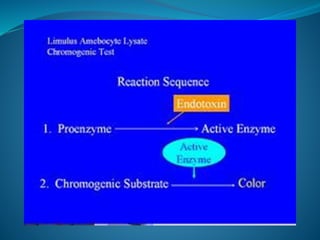
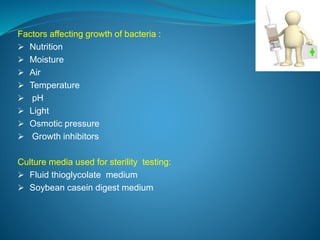
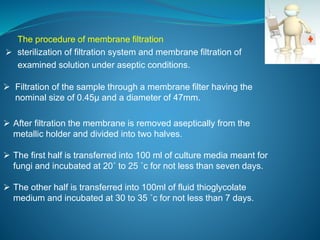
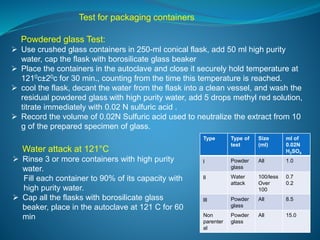
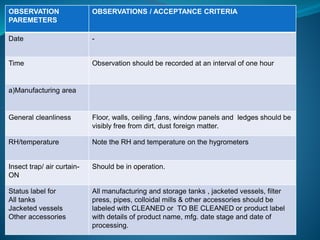
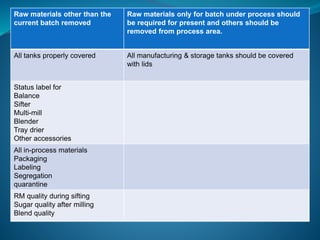
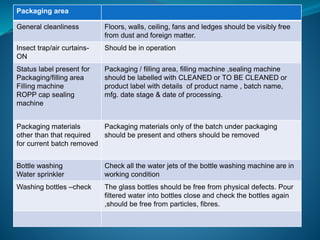
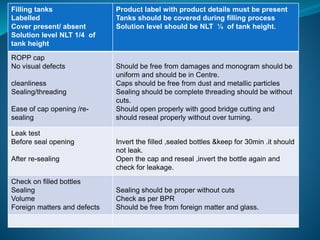
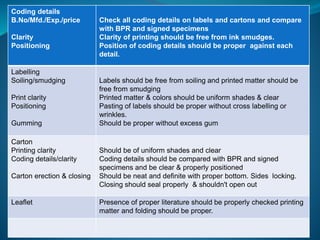
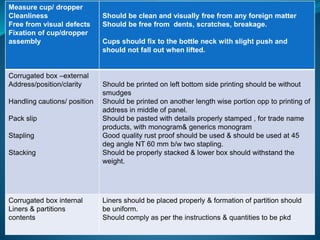
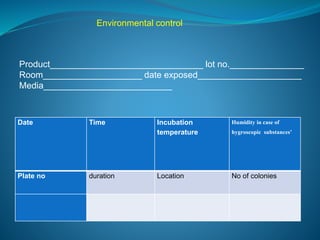
The document discusses in-process quality control (IPQC) for parenteral products. IPQC involves controlling manufacturing procedures from raw materials to finished product release. Key IPQC tests for parenterals include clarity testing to detect particulate matter using visual or automated methods, leakage testing of packaging, testing fill volume and pH, and sterility testing. The document outlines various physical, chemical, biological, and microbiological tests performed during IPQC to ensure product quality.