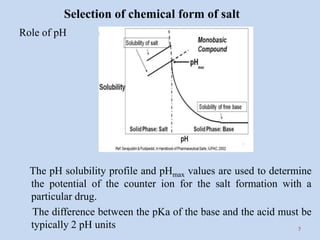
This document discusses salt selection strategies in pharmaceutical product development. It begins by explaining the importance of salt formation in improving drug solubility, permeability, and dissolution. The key criteria for salt selection are discussed, including aqueous solubility, crystallinity, hygroscopicity, stability, and ease of synthesis. A multi-tiered approach is described for selecting the chemical form and physical form of the salt. A case study demonstrates evaluating different salt forms of a drug and selecting the optimal sulfate salt. The conclusion emphasizes that a multidisciplinary team approach and proper screening strategies are important for efficiently selecting the best salt form early in development.