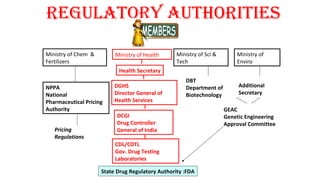
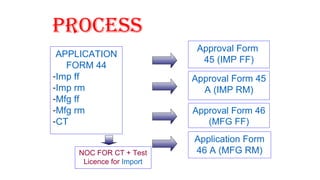
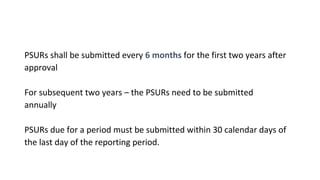
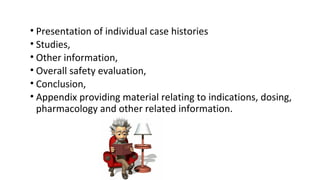
This document provides guidelines for conducting clinical trials and obtaining regulatory approval for new drugs in India. It outlines the requirements and process for importing or manufacturing new drugs, including application forms, fees, pre-clinical and clinical data requirements, and responsibilities of sponsors, investigators and ethics committees. Key points covered include Schedule Y which provides Good Clinical Practice guidelines; types of clinical trials and special population studies; and the roles of regulatory authorities like the Drug Controller General of India.