This document provides an overview of preformulation studies, which characterize the physical and chemical properties of new drug molecules to aid in the development of safe, effective, and stable dosage forms. Some key points covered include:
- Preformulation studies give direction for dosage form selection, excipient choice, composition, and process development.
- Important physicochemical properties to determine include solubility, partition coefficient, pKa, stability, and interactions with excipients.
- Methods are described for evaluating properties like solubility, dissolution, oxidation, hydrolysis, and polymorphism which can impact stability and bioavailability.
- Understanding these properties aids in developing robust formulations and setting appropriate storage conditions for drug products





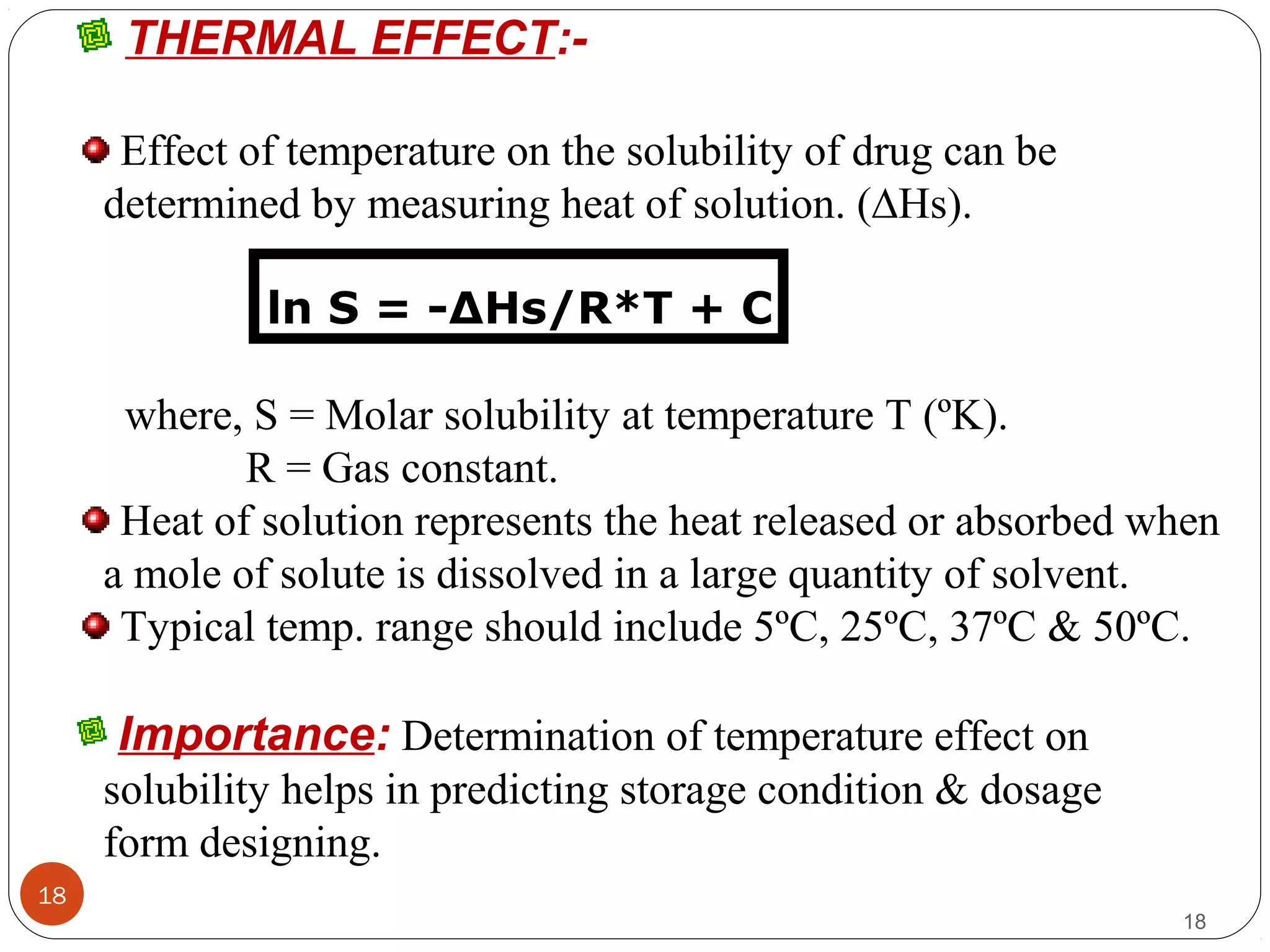
![aQUeoUs solUBility:-
There are two fundamental properties mandatory for a new
compound.
(A) INTRINSIC SOLUBILITY (CO) :-
9
S = So {1 + (K1 / [H+])} ------ for weak acids.
S = So {1 + ([H+] / K2)} ------ for weak bases.
where, S = Solubility at a given pH.
So = Intrinsic solubility of the neutral form.
K1 = Dissociation constant of weak acid.
K2= Dissociation constant of weak base.
9Contd…](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-9-2048.jpg)

![(B) Ionization constant (pKa):-
11
75 % of all drugs are weak bases,
20 % are weak acids and only,
5 % are nonionic amphoteric or alcohol.
Henderson-Hasselbalch equation:-
pH = pKa + log [ionized form] / [unionized form] --- for acids.
pH = pKa + log [unionized form] / [ionized form] --- for bases.
Uses of these equations:-
To determine pKa.
To predict solubility at any pH provided that Co & pKa are
known.
To facilitate the selection of suitable salt forming compounds.
To predict the solubility & pH properties of the salts.
11](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-11-2048.jpg)



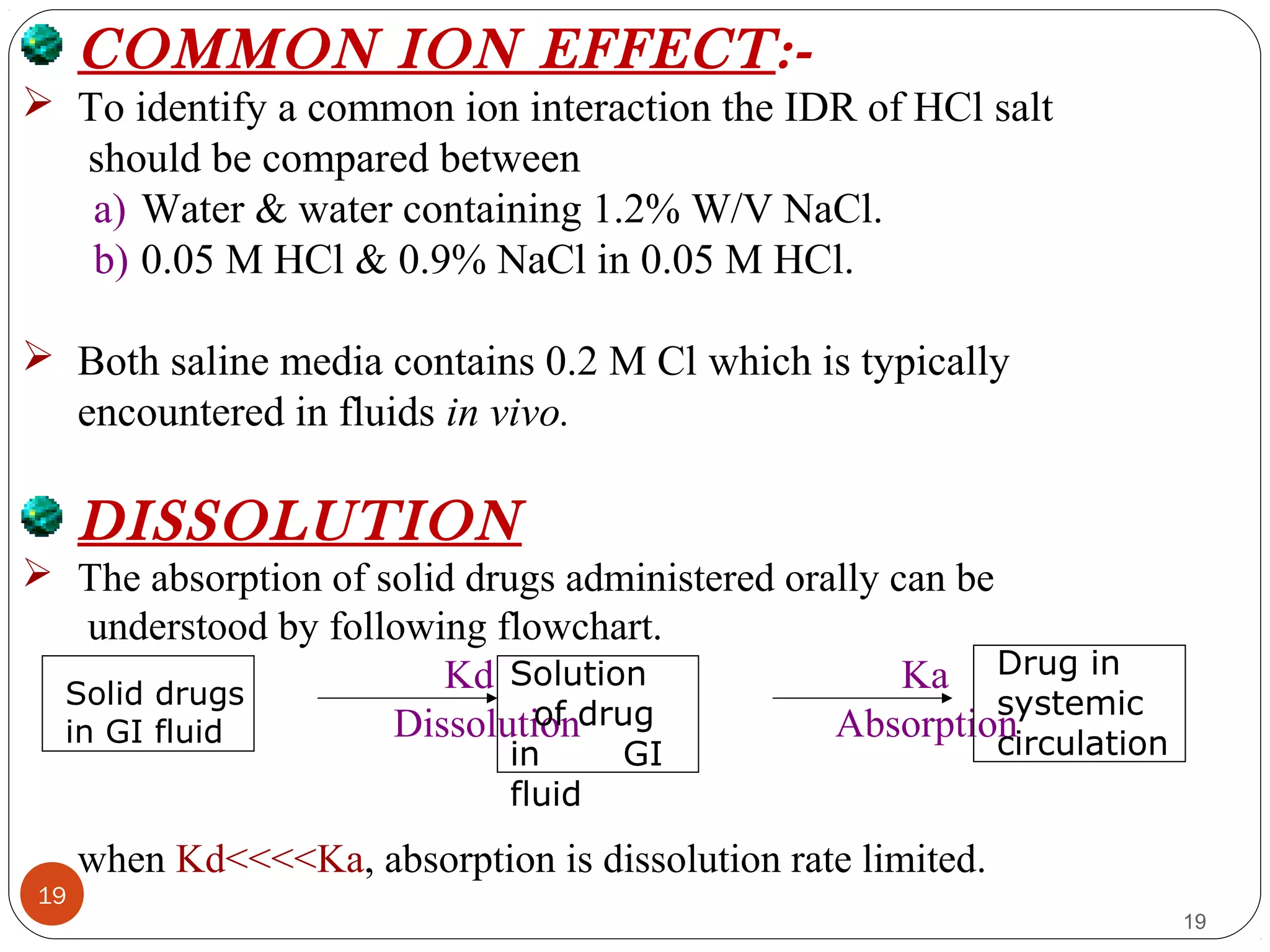

![21
21
Particulate dissolution
Determine the dissolution of solids at different surface area.
It is used to study the influence on dissolution of particle size,
surface area & mixing with excipients.
STABILITY ANALYSIS
Development of a drug substance into a suitable dosage form
requires the preformulation stability studies as:
[1] Solid state stability.
[2] Solution state stability.
SOLID STATE STABILITY:-
Solid state reactions are much slower & more difficult to
interpret than solution state reactions because of reduced no.
of molecular contacts between drug & excipient molecules &
occurrence of multiple reactions.](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-21-2048.jpg)






![28
28
pREvENTION OF OXIDATION:-
1)Reducing oxygen content.
2)Storage in a dark & cool condition.
3)Addition of chelating agent. [Eg. EDTA, Citric acid, Tartaric
acid].
4)Adjustment of pH.
5)Changing solvent. [Eg. Aldehydes, ethers, ketones may
influence free radical reaction].
6)Addition of an antioxidant.
a.Reducing agent.
b.Chain inhibitors of radical induced decomposition](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-28-2048.jpg)




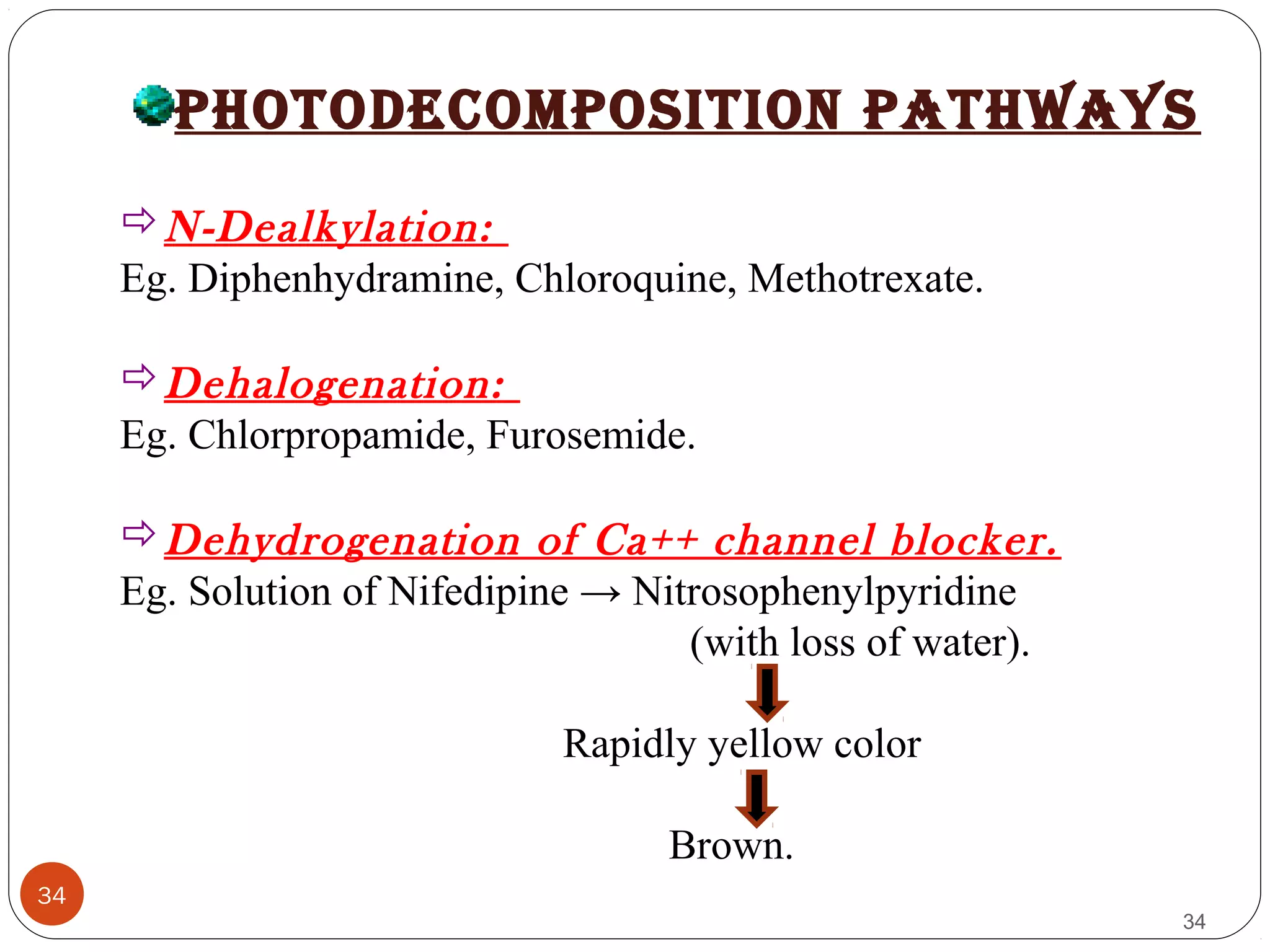



![38
38
Avoiding sunbath.
Eg. Sparfloxacin.
Photostabilizer [Light absorber].
• Colorant Curcumine, Azorubine.
• Pigments Iron oxide, Titanium dioxide.
Coating:
Pigments like TiO2(IN NIFEDIPINE) / ZnO.
Eg. Photostabilization of Sulphasomidine Tab. by film
coating containing U.V. absorber (Oxybenzone) to protect
color & photolytic degradation.](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-38-2048.jpg)

![40
40
PoLYmeriZAtion
It is a continuous reaction between molecules.
More than one monomer reacts to form a polymer.
Eg. Darkening of glucose solution is attributed to polymerization
of breakdown product [5- (hydroxyl methyl) furfural].
Eg. Shellac on aging undergoes polymerization & hence
prolongs disintegration time &dissolution time.](https://image.slidesharecdn.com/preform3-190218165914/75/preformulation-study-40-2048.jpg)