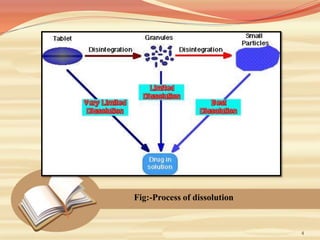
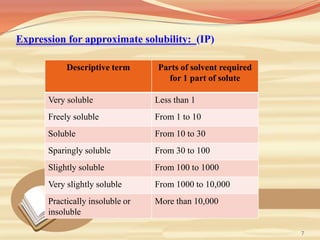
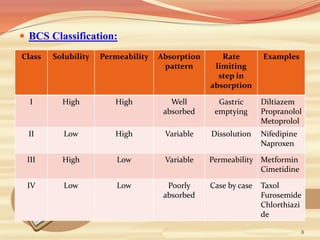
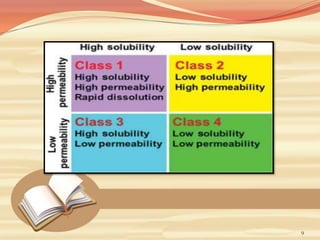
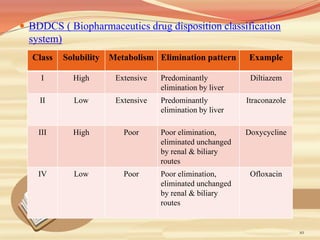
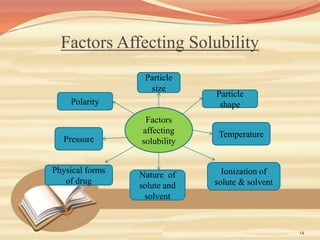
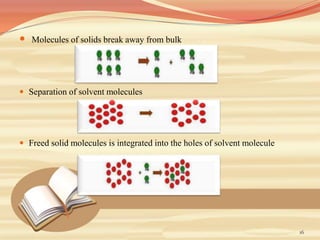
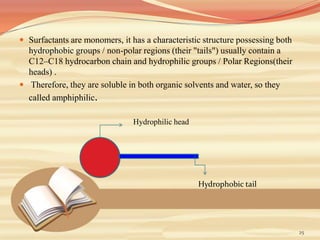
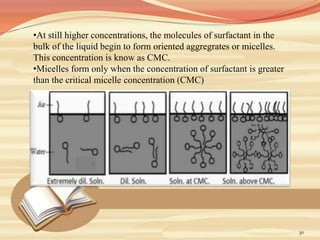
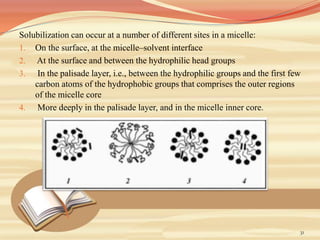
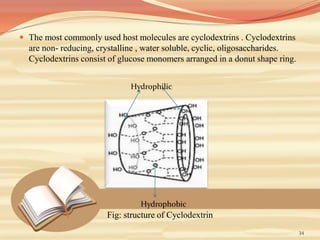
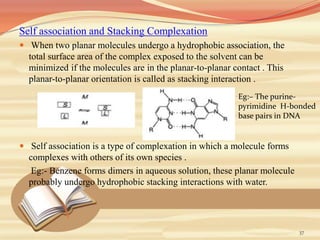
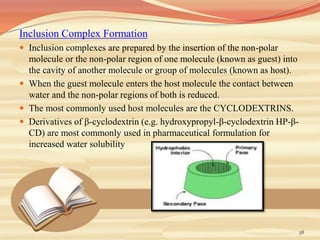
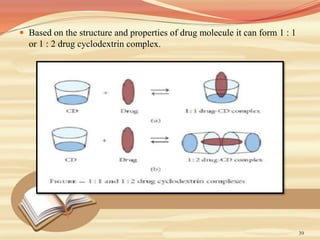
This document provides an overview of solubility and dissolution. It defines key terms like solubility, dissolution, and saturation solubility. It discusses factors that affect solubility like temperature, particle size, and polarity. It also describes different techniques to improve solubility including use of co-solvents, surfactants, and complexation. Surfactants can improve solubility through micelle formation. The document is intended as an introduction to solubility and techniques to enhance solubility of poorly soluble drugs.