The document describes various tests conducted on pharmaceutical samples, including:
- Weight/ml and density tests to determine the weight of a liquid per milliliter.
- Total solids tests to determine the residue left after drying a sample.
- Ash testing to determine acid soluble, acid insoluble, water soluble and sulphated ash contents.
- Toxicity tests conducted on finished drug products and packaging to assess safety.
- Loss on drying tests to determine volatile content lost after drying under specified conditions.
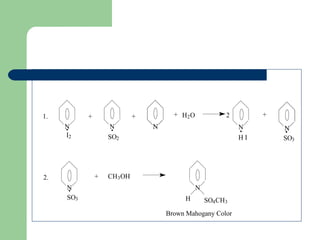
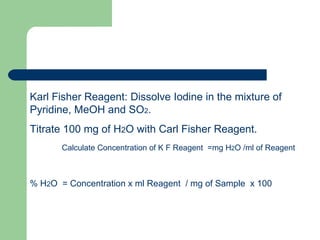
- Moisture content tests using thermogravimetric analysis or Karl Fischer titration.