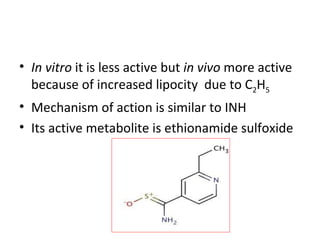
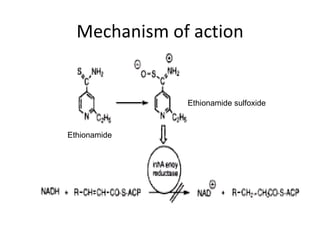
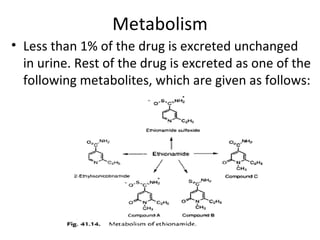
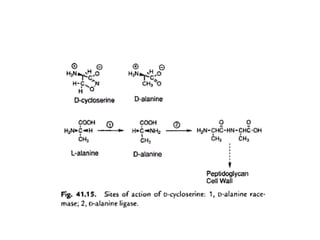
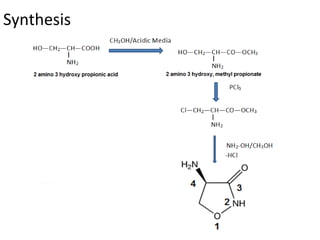
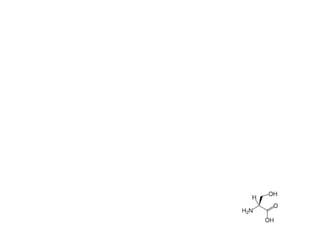
This document discusses the anti-tuberculosis drug ethionamide. It is a second-line drug that is an analogue of isonicotinamide but contains sulfur instead of oxygen. It is less active in vitro but more active in vivo due to increased lipophilicity from its ethyl group. Its mechanism of action involves conversion to its active metabolite ethionamide sulfoxide via oxidation, which then inactivates the inhA enoyl reductase enzyme. Less than 1% is excreted unchanged in urine with the rest excreted as metabolites.