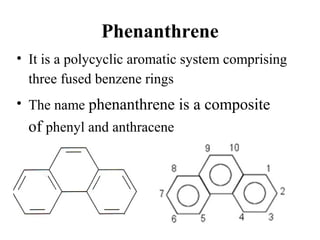
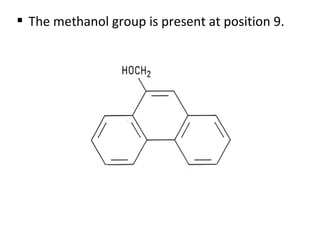
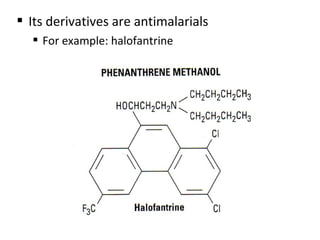
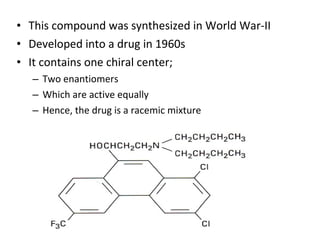
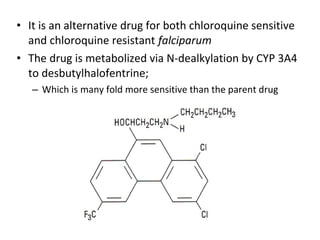
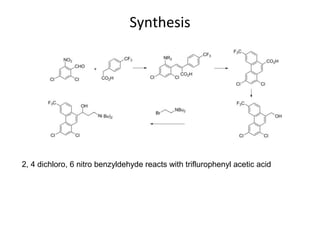
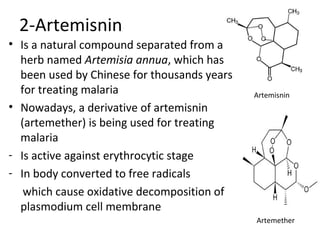
This document discusses 9-phenethrenyl methanol class derivatives that are used as antimalarial drugs, specifically halofantrine. It notes that halofantrine contains one chiral center and is administered as a racemic mixture. It is an alternative treatment for chloroquine sensitive and resistant malaria. However, it can cause serious heart problems and its effectiveness is limited by low bioavailability and development of resistance due to its long half-life. The document also briefly discusses the natural antimalarial compound artemisinin and its derivative artemether, noting its mechanism of action involves free radicals produced from its unusual peroxide bridge.