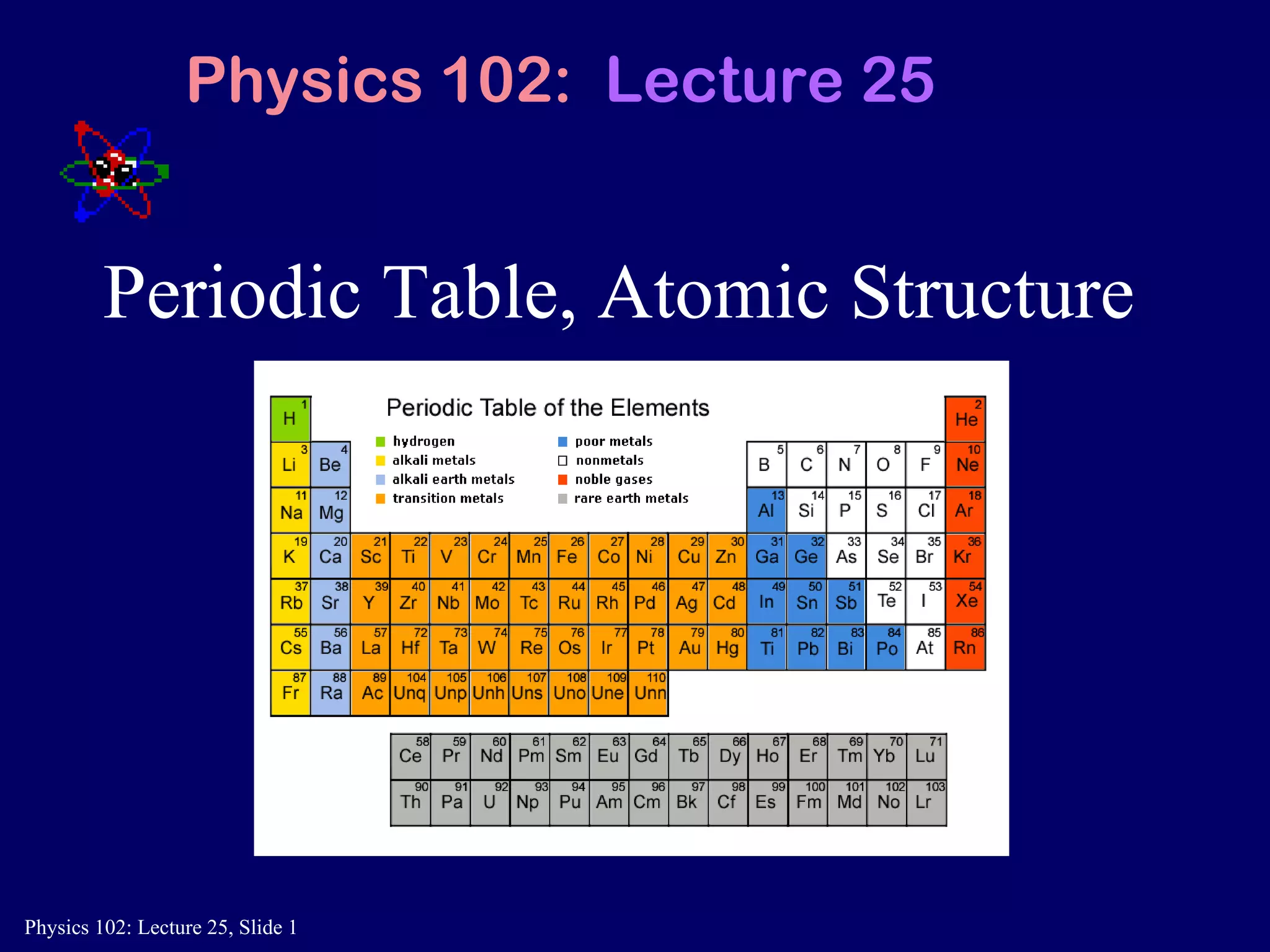
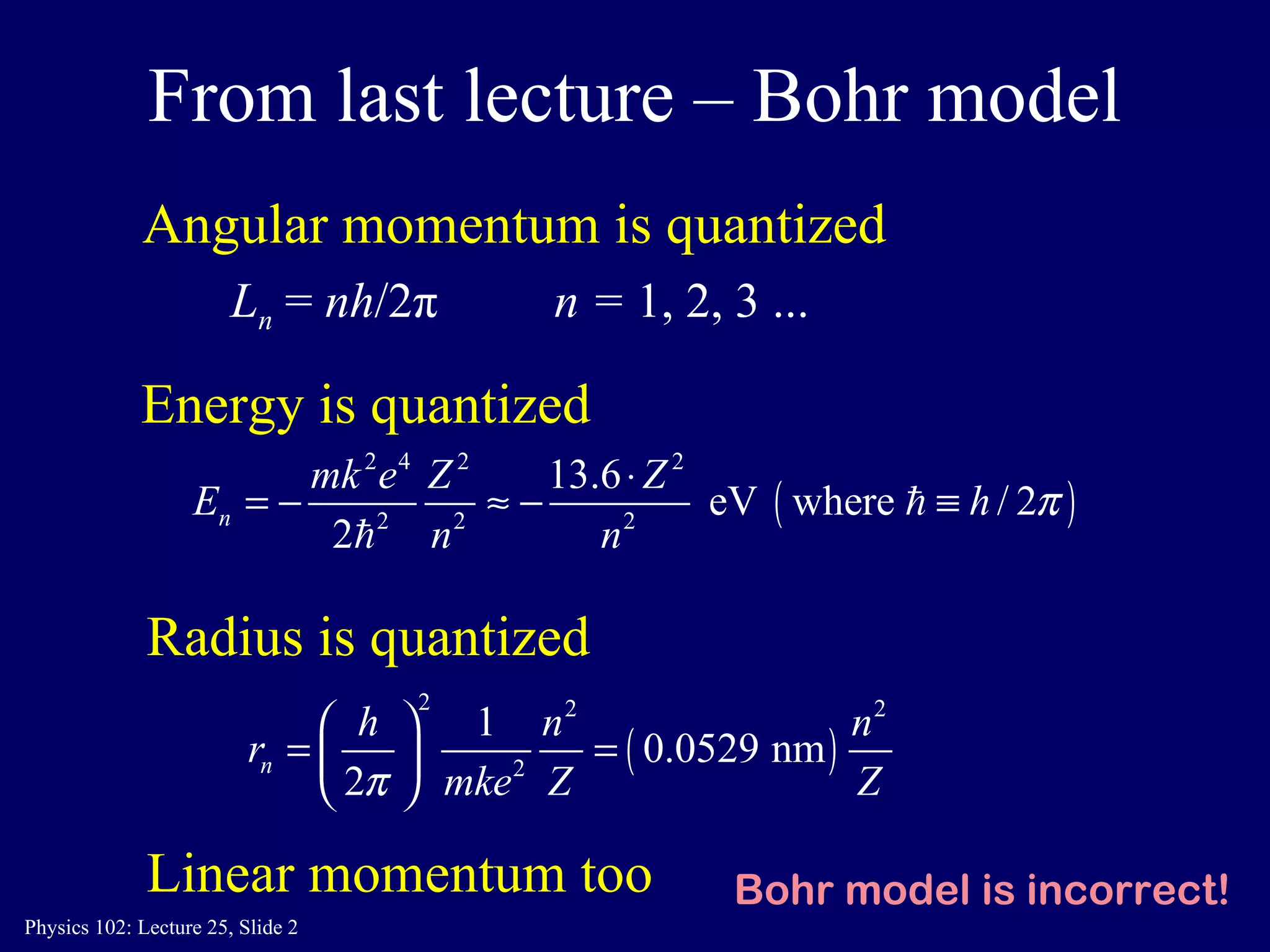
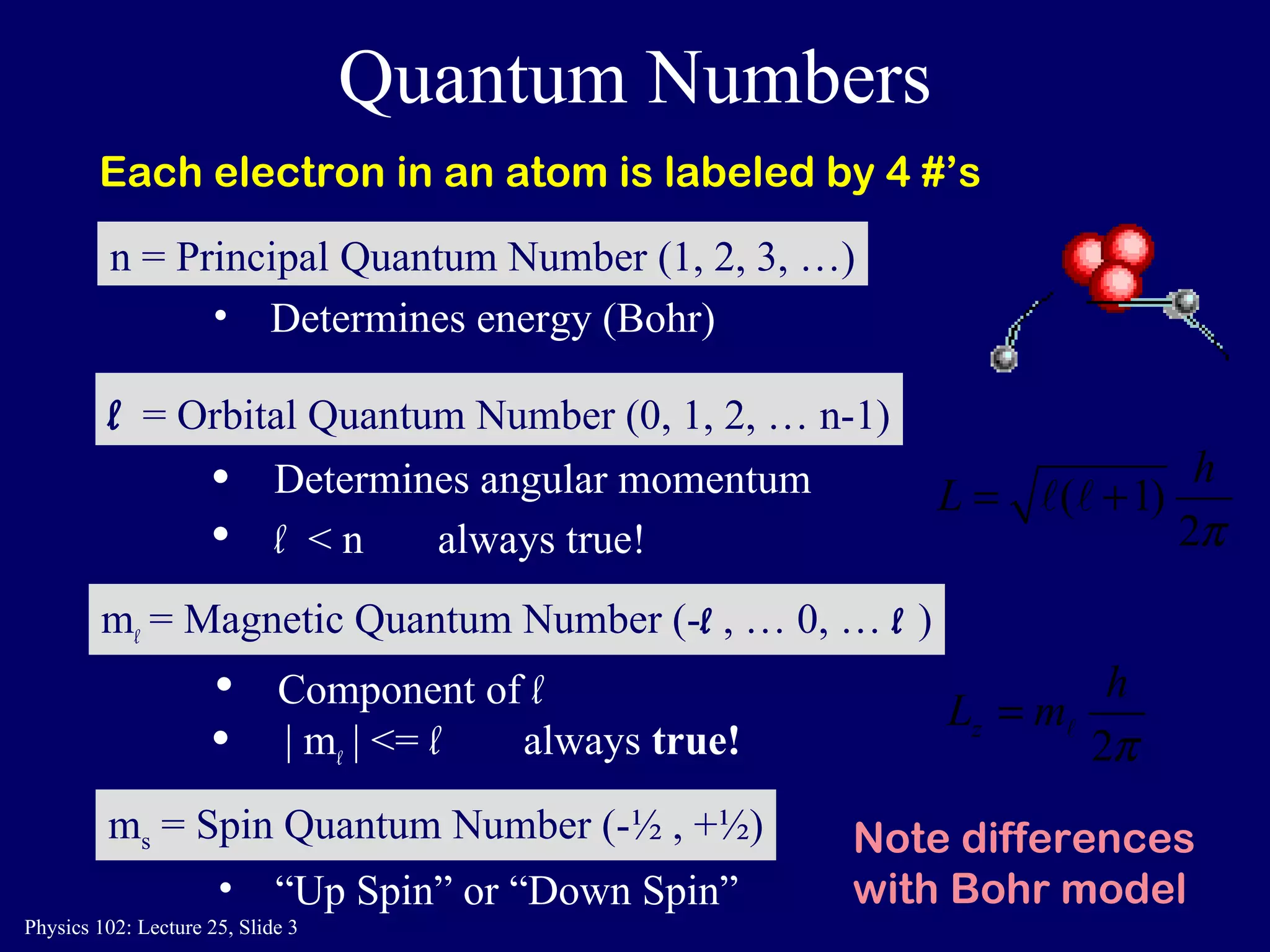
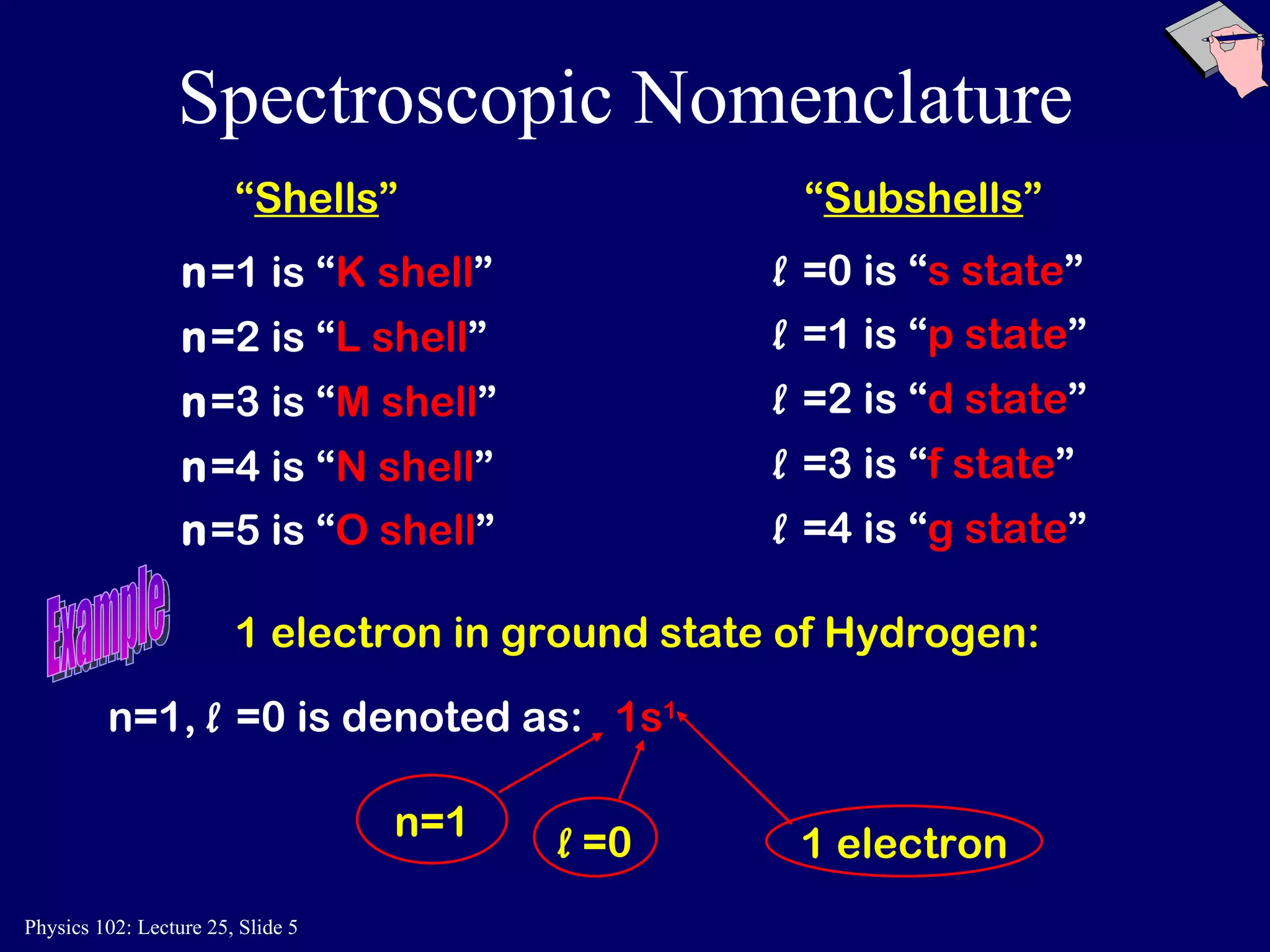
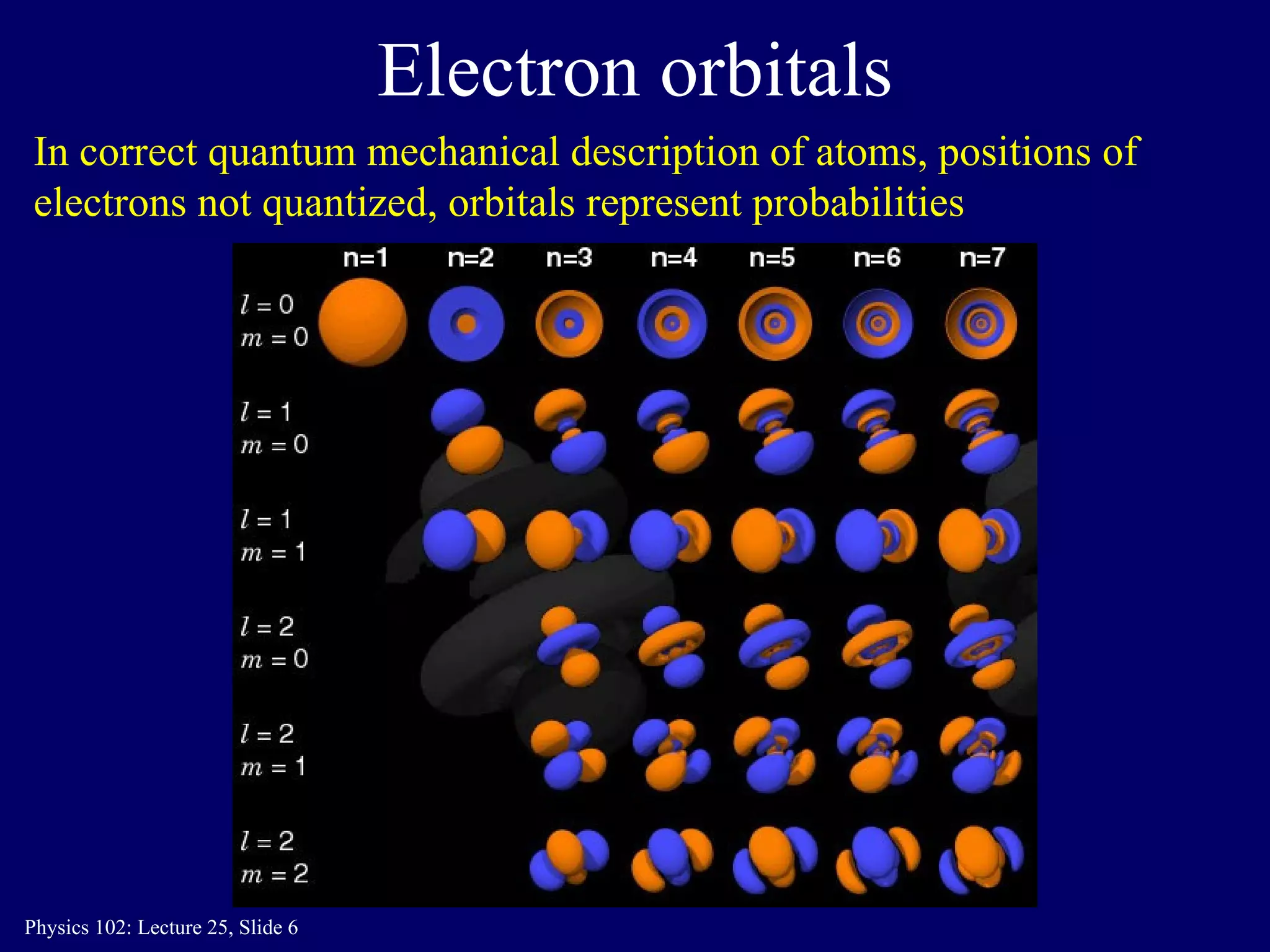
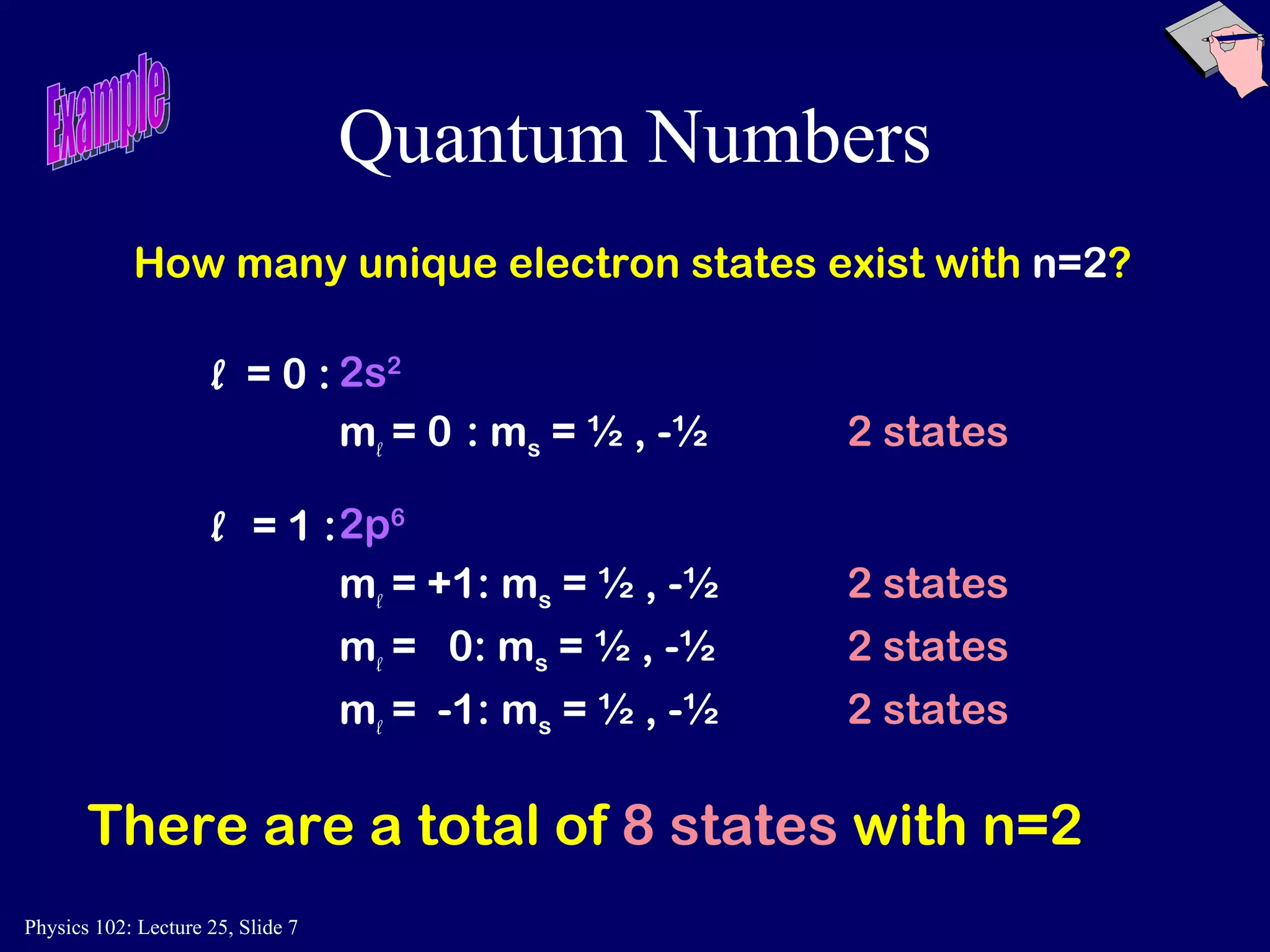
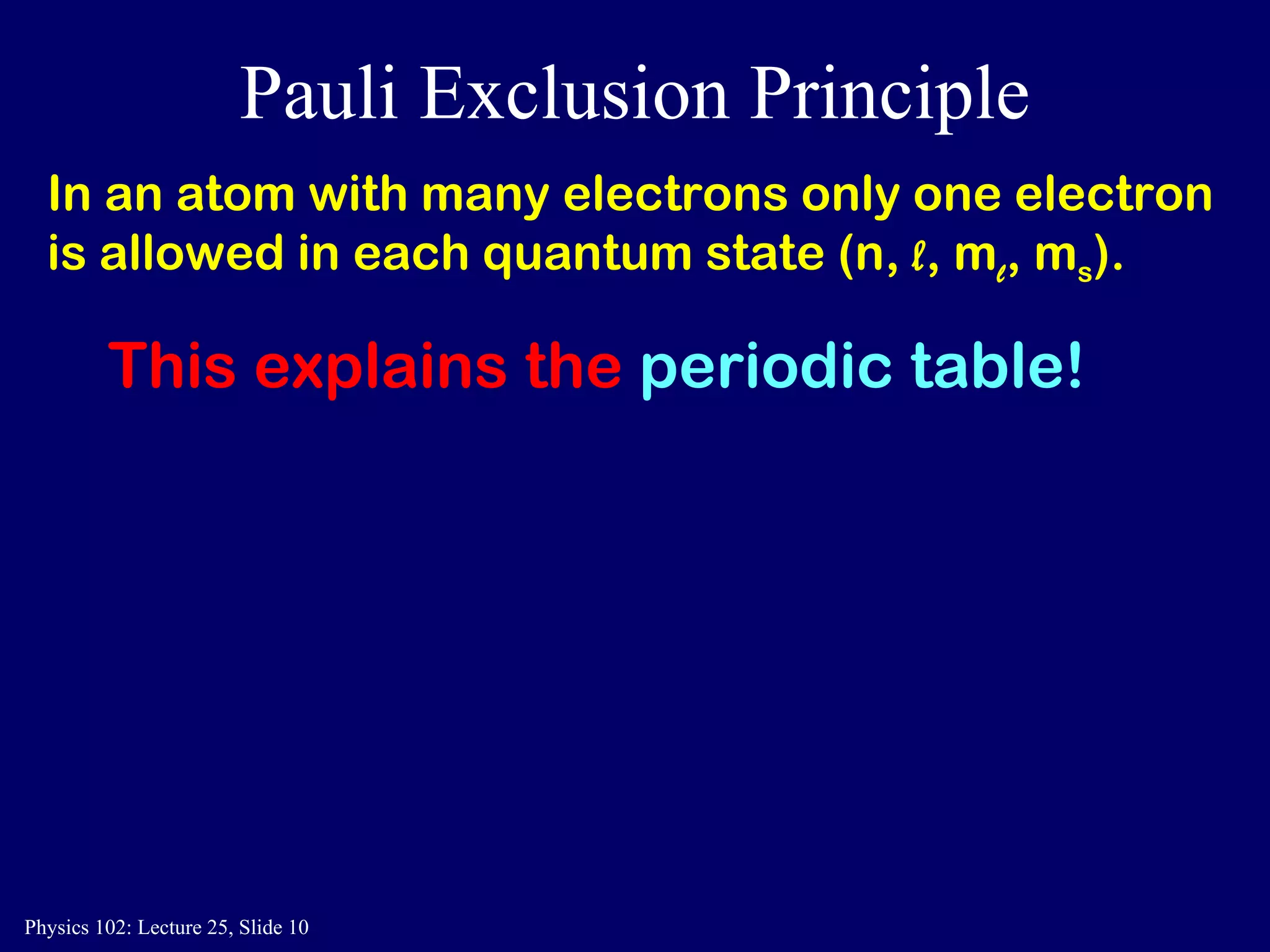
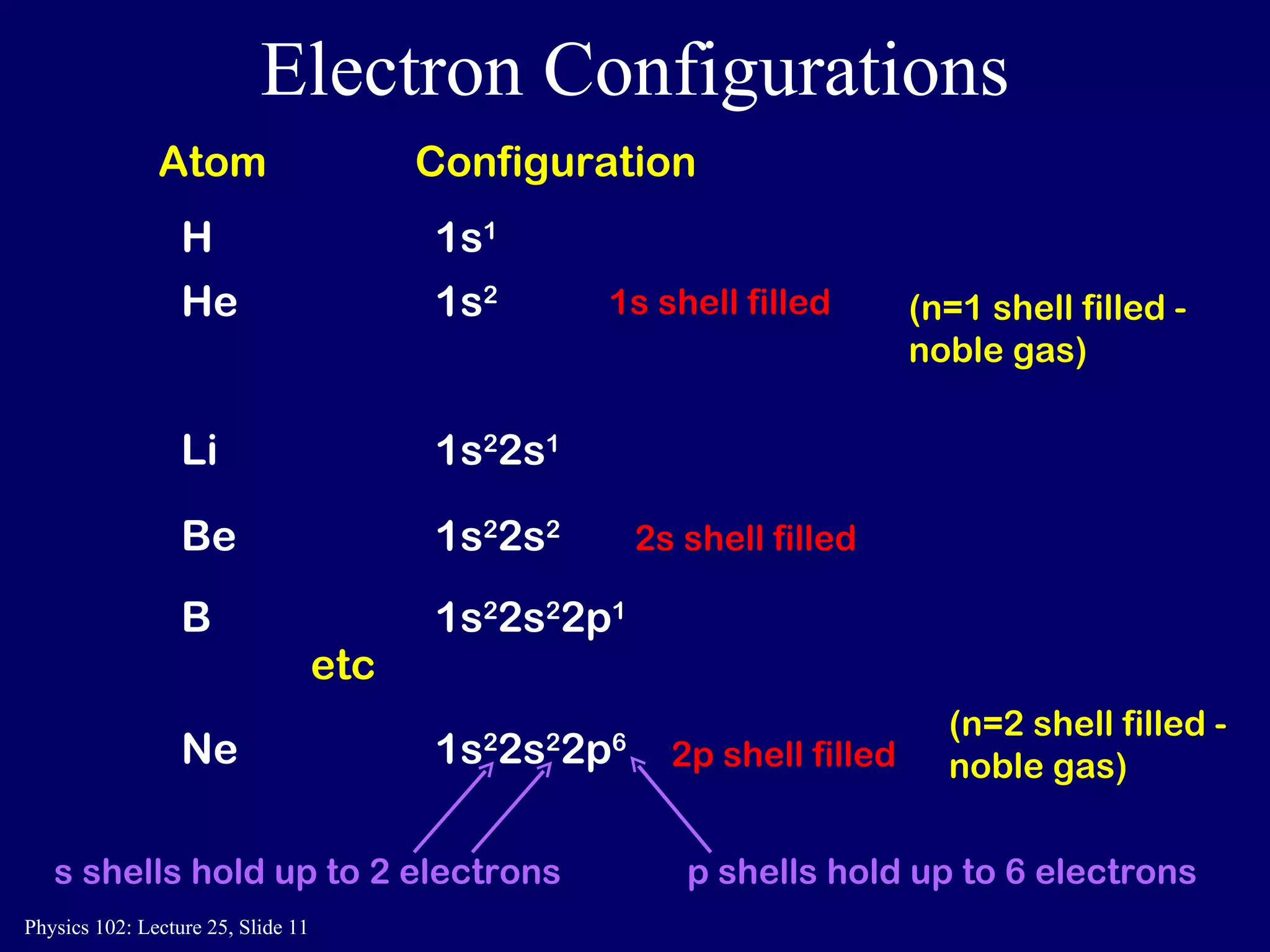
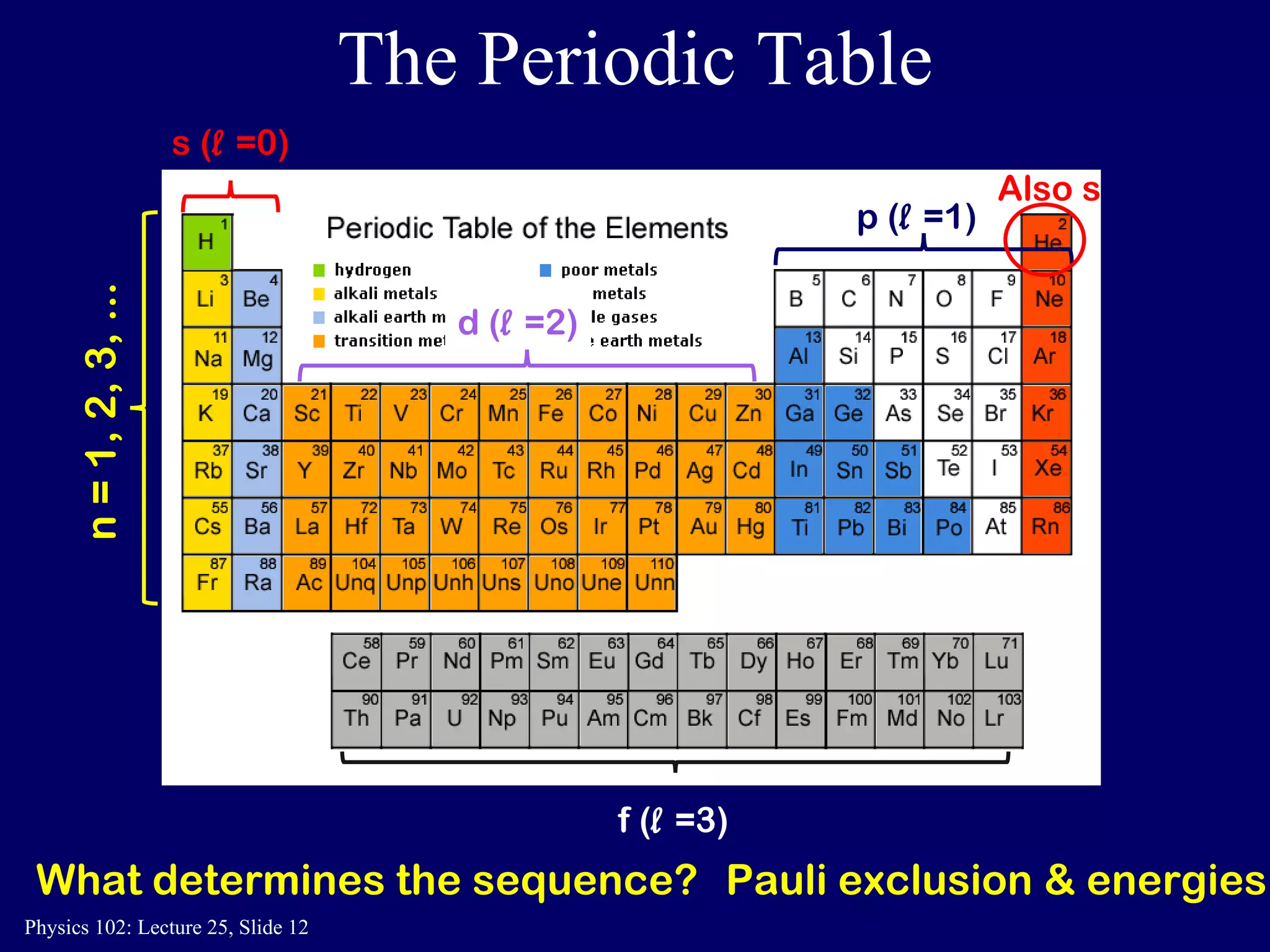
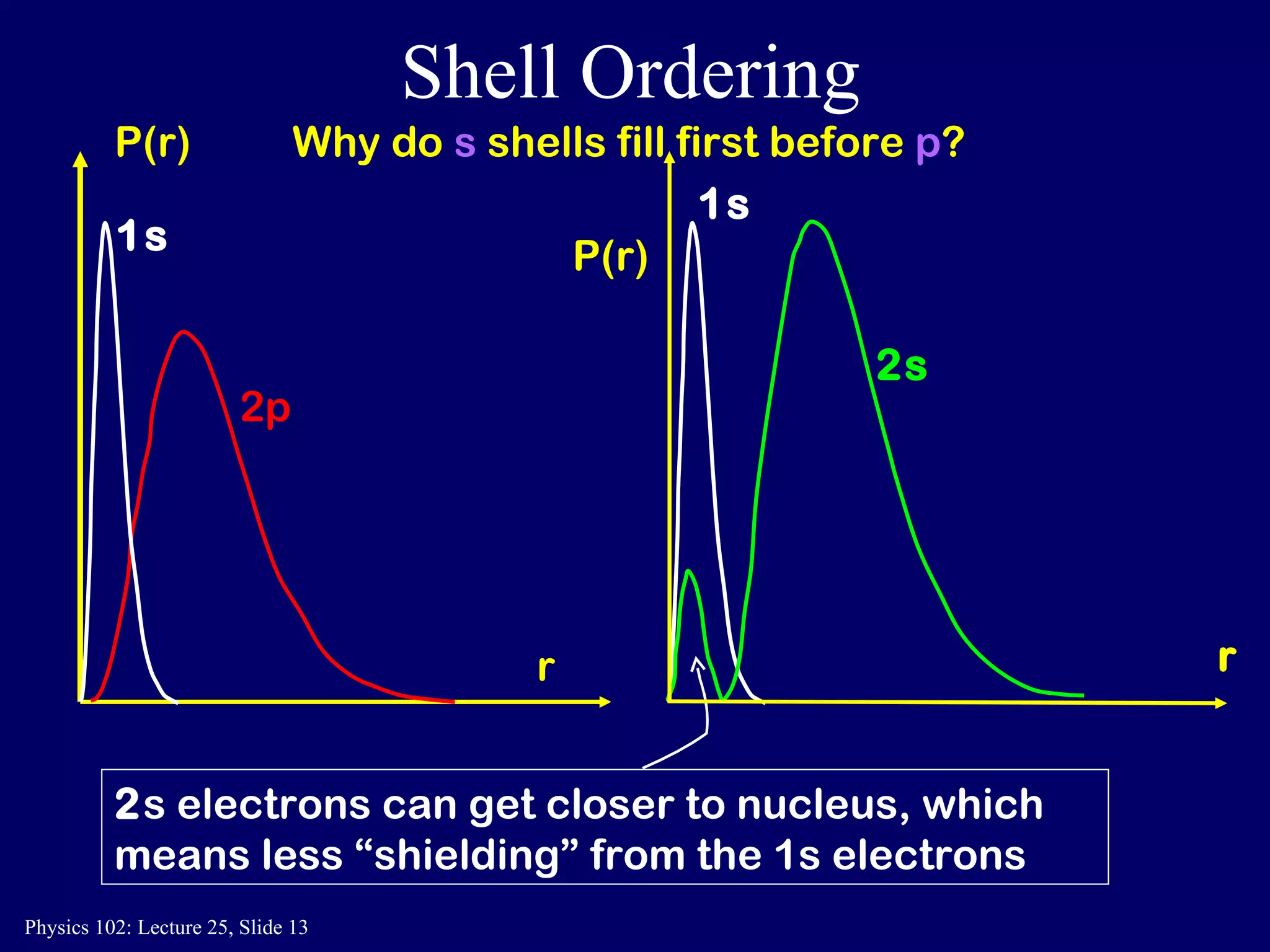
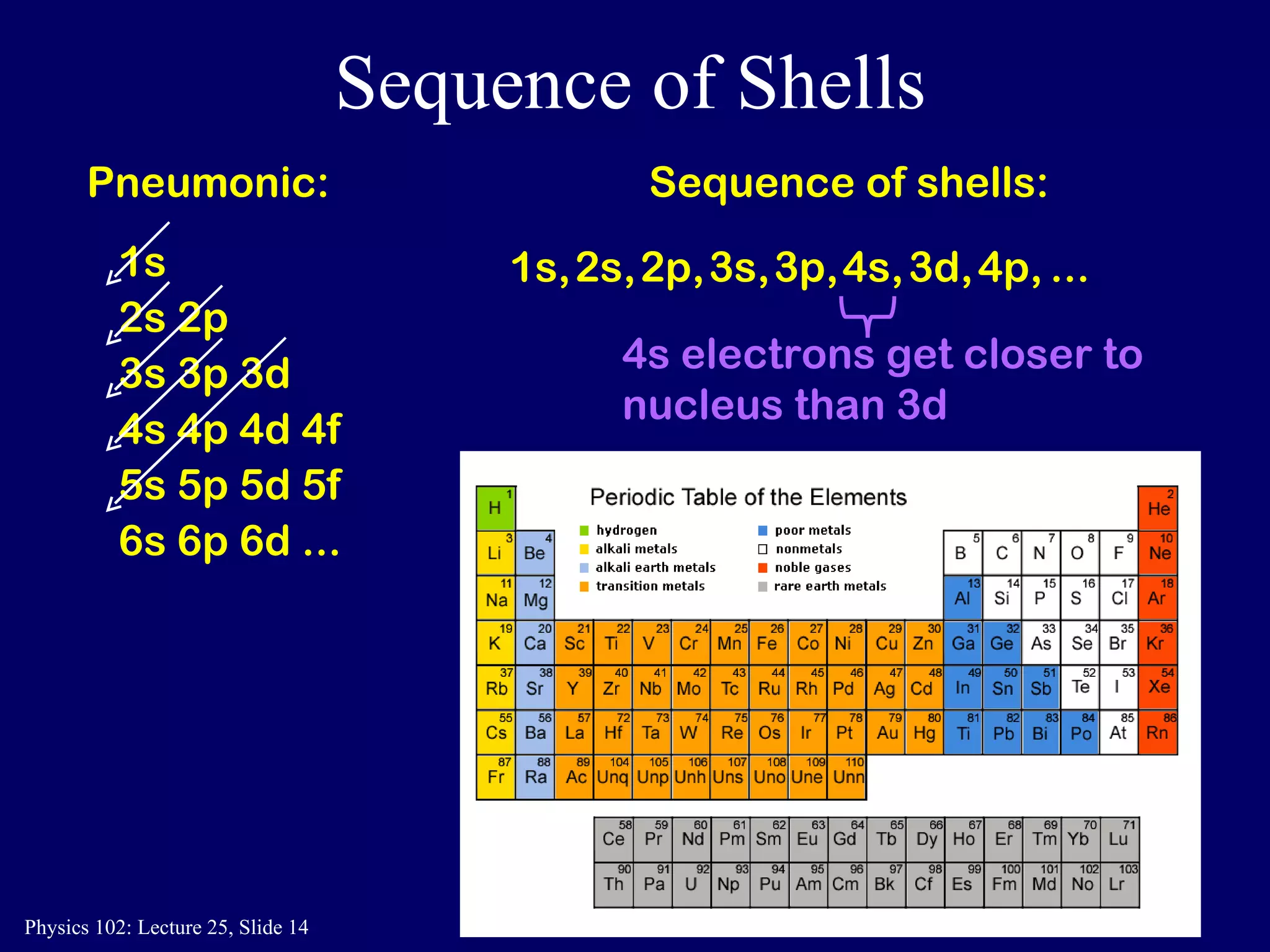
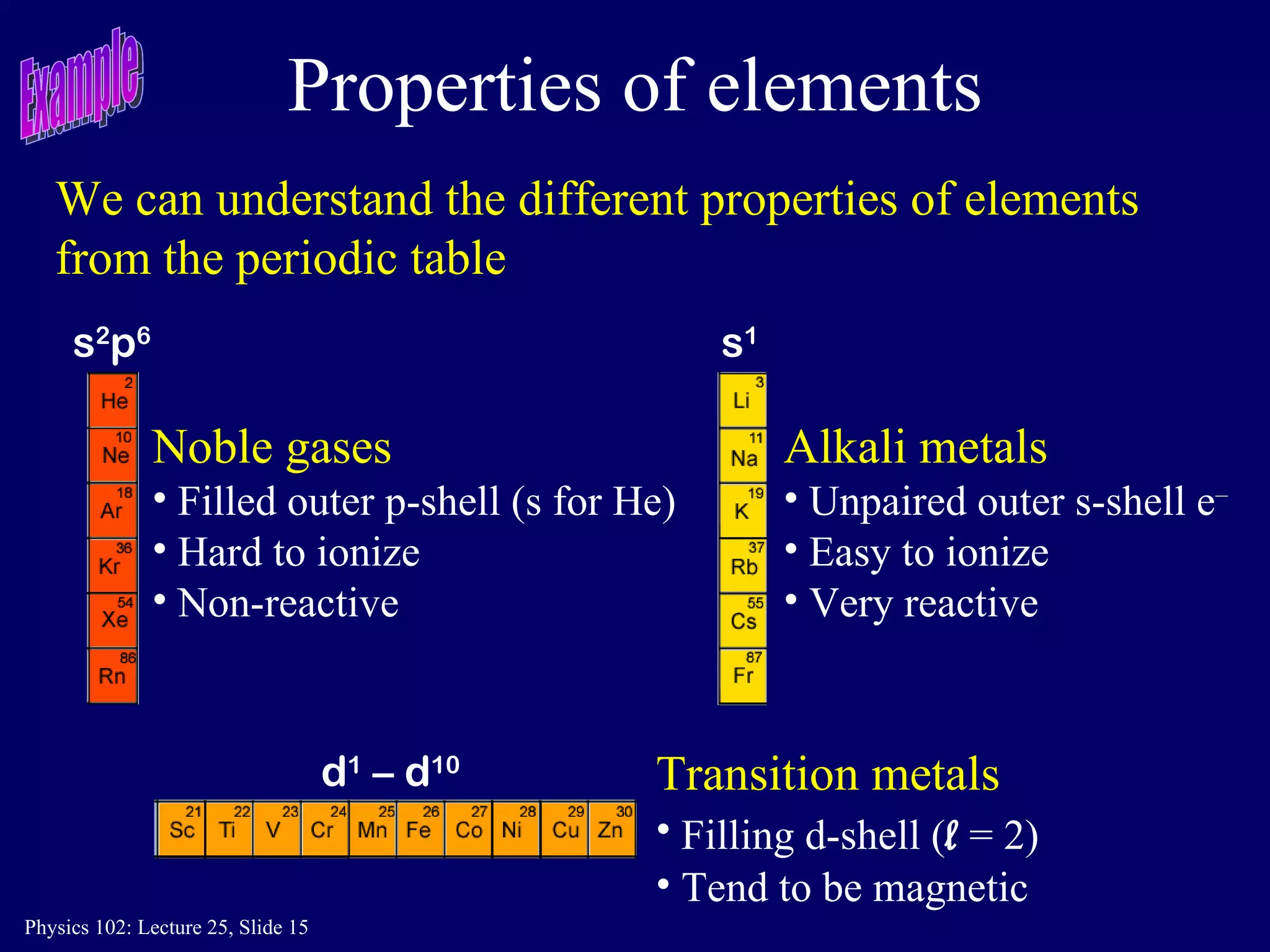
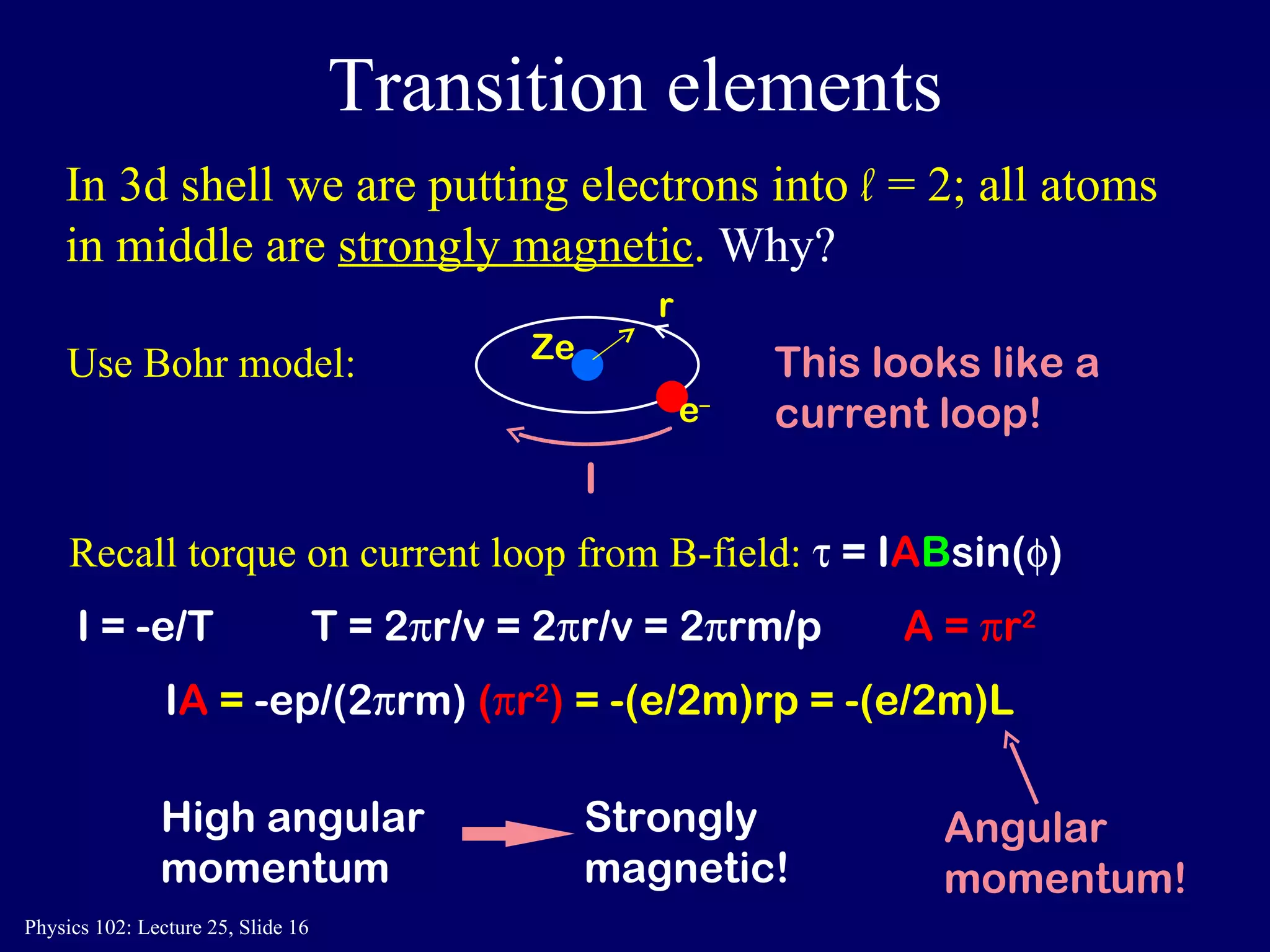
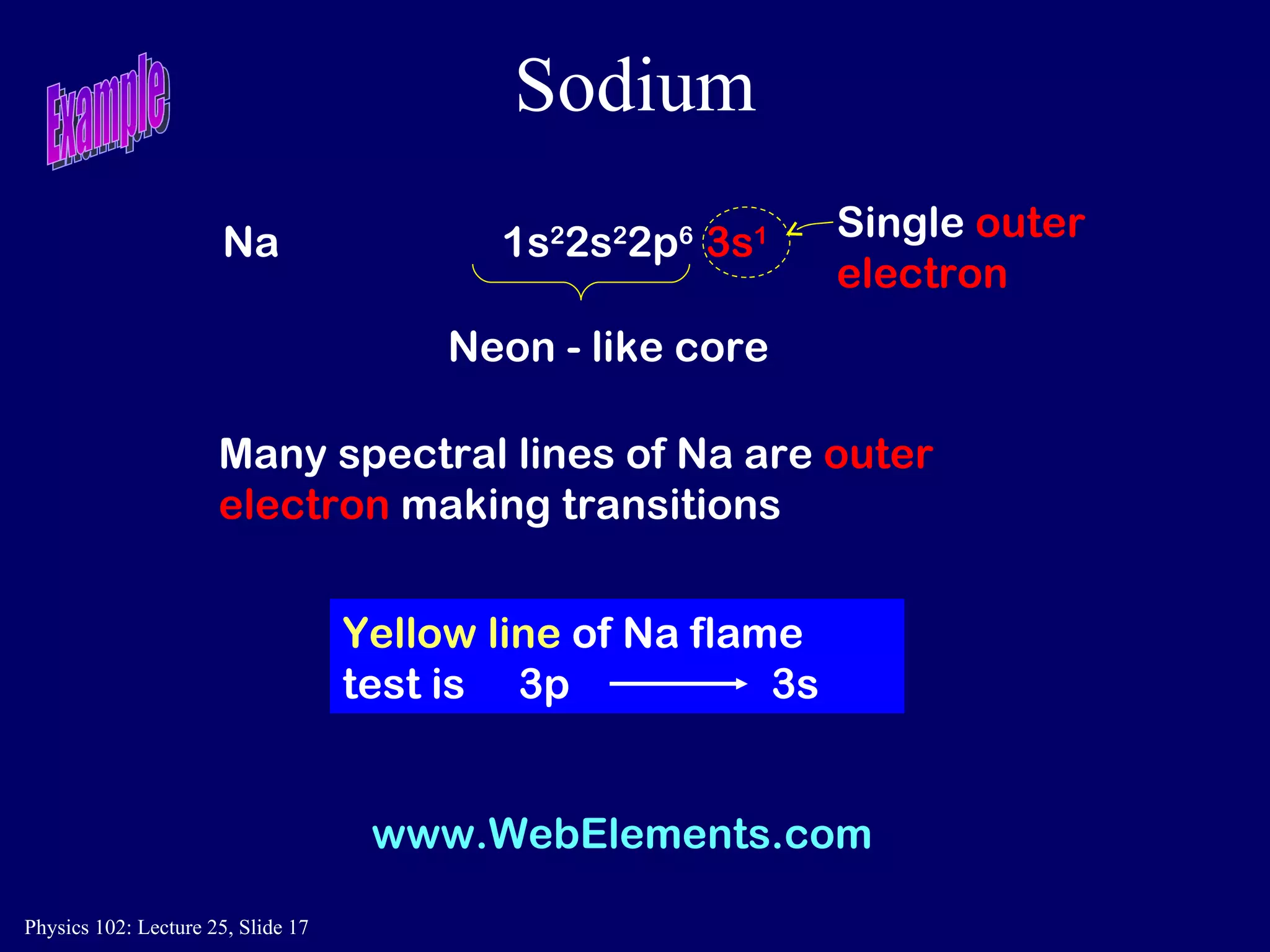
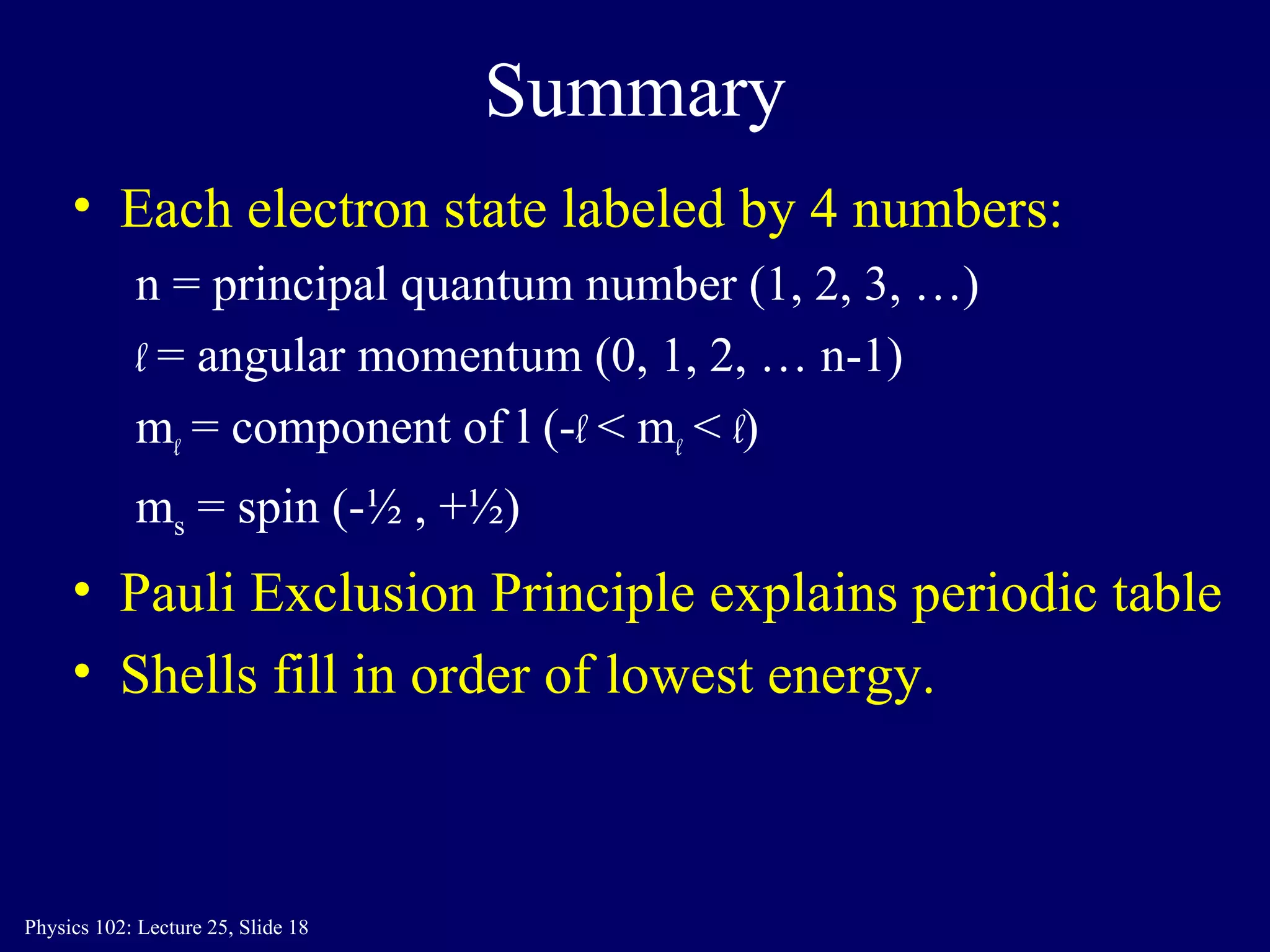
The document discusses the quantum numbers that describe the electron states in an atom. It explains that each electron is labeled by four quantum numbers: principal (n), angular momentum (l), magnetic (ml), and spin (ms). The Pauli Exclusion Principle states that each quantum state can contain only one electron, which explains the filling order and structure of the periodic table.