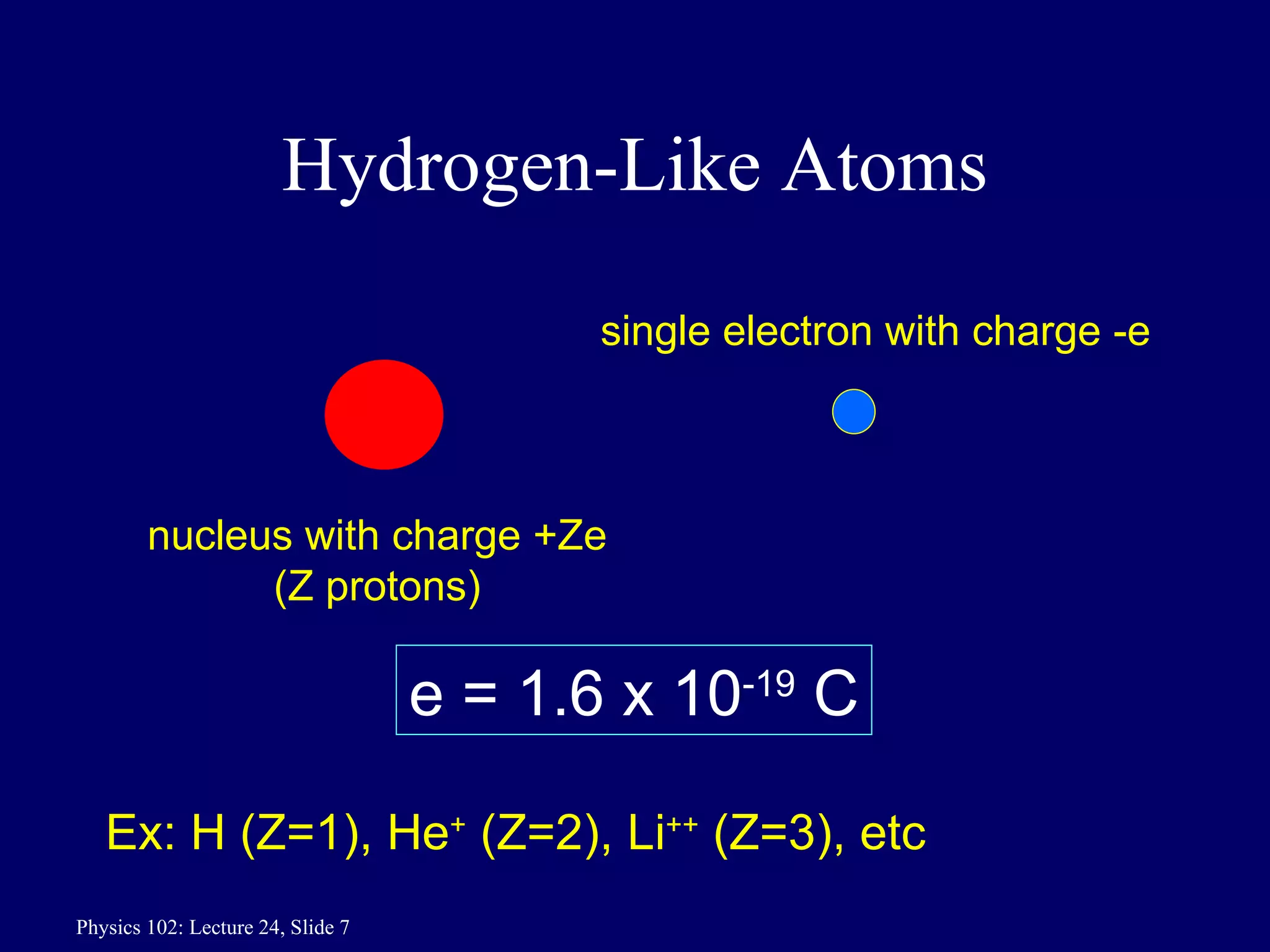
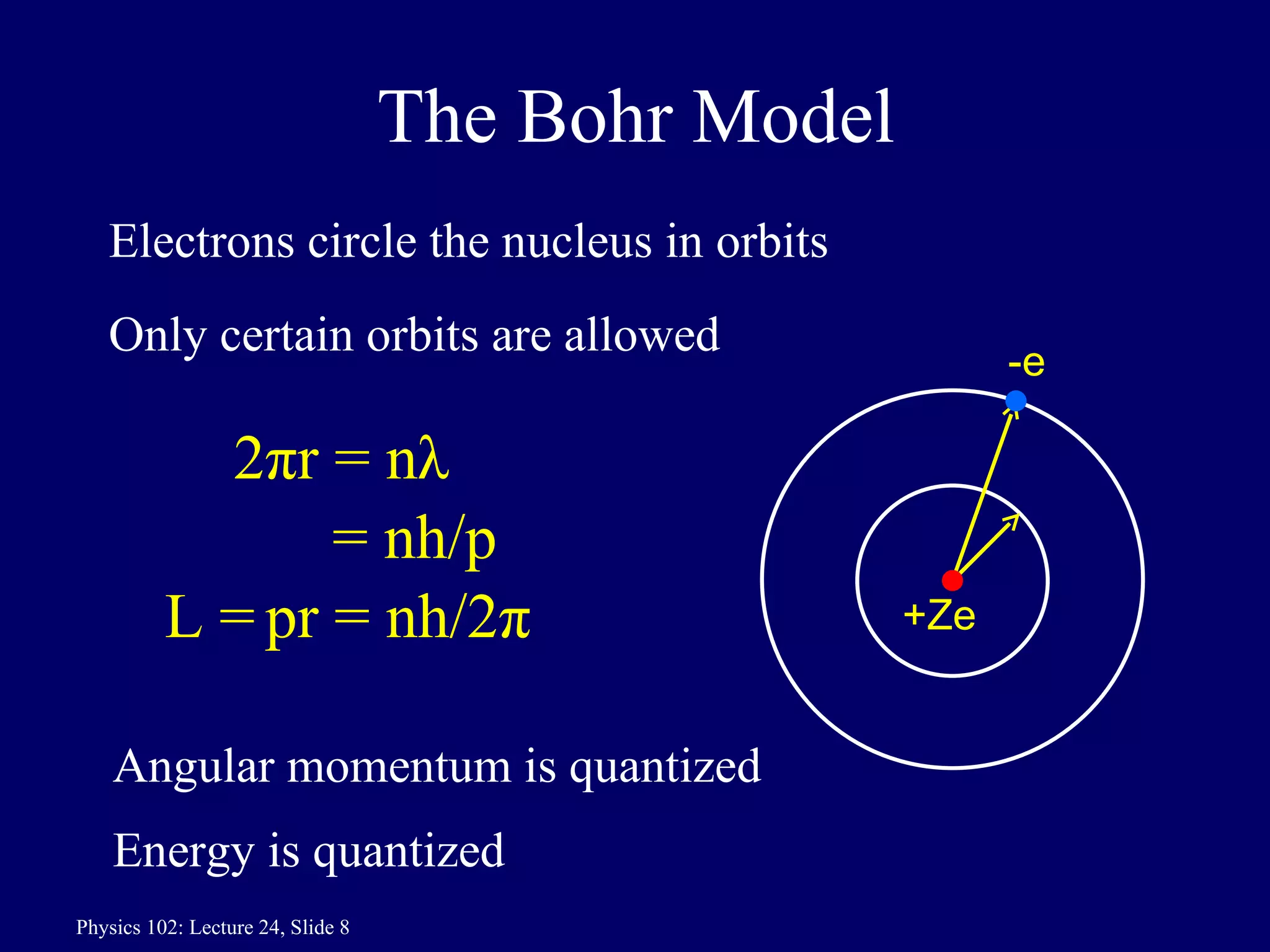
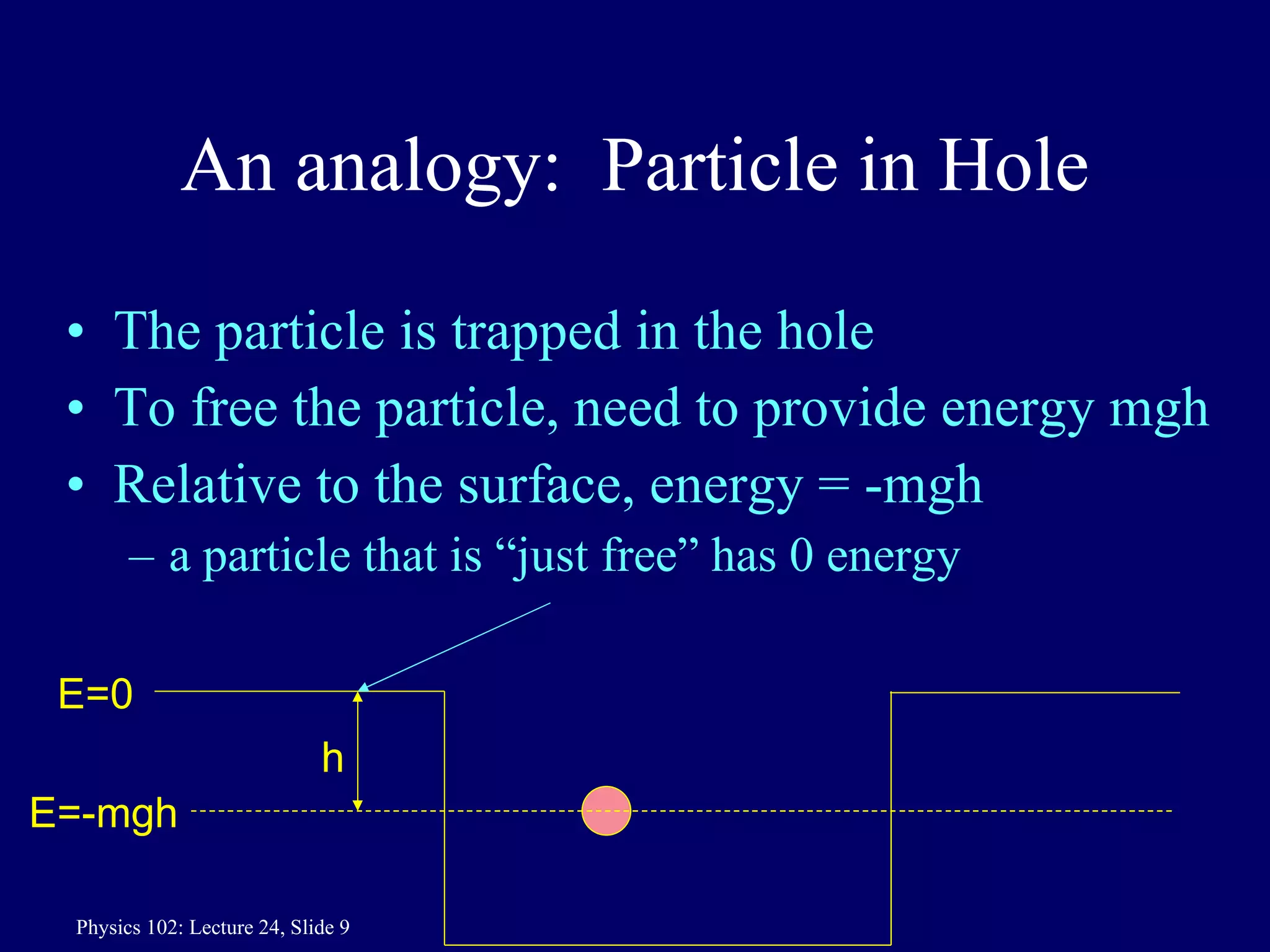
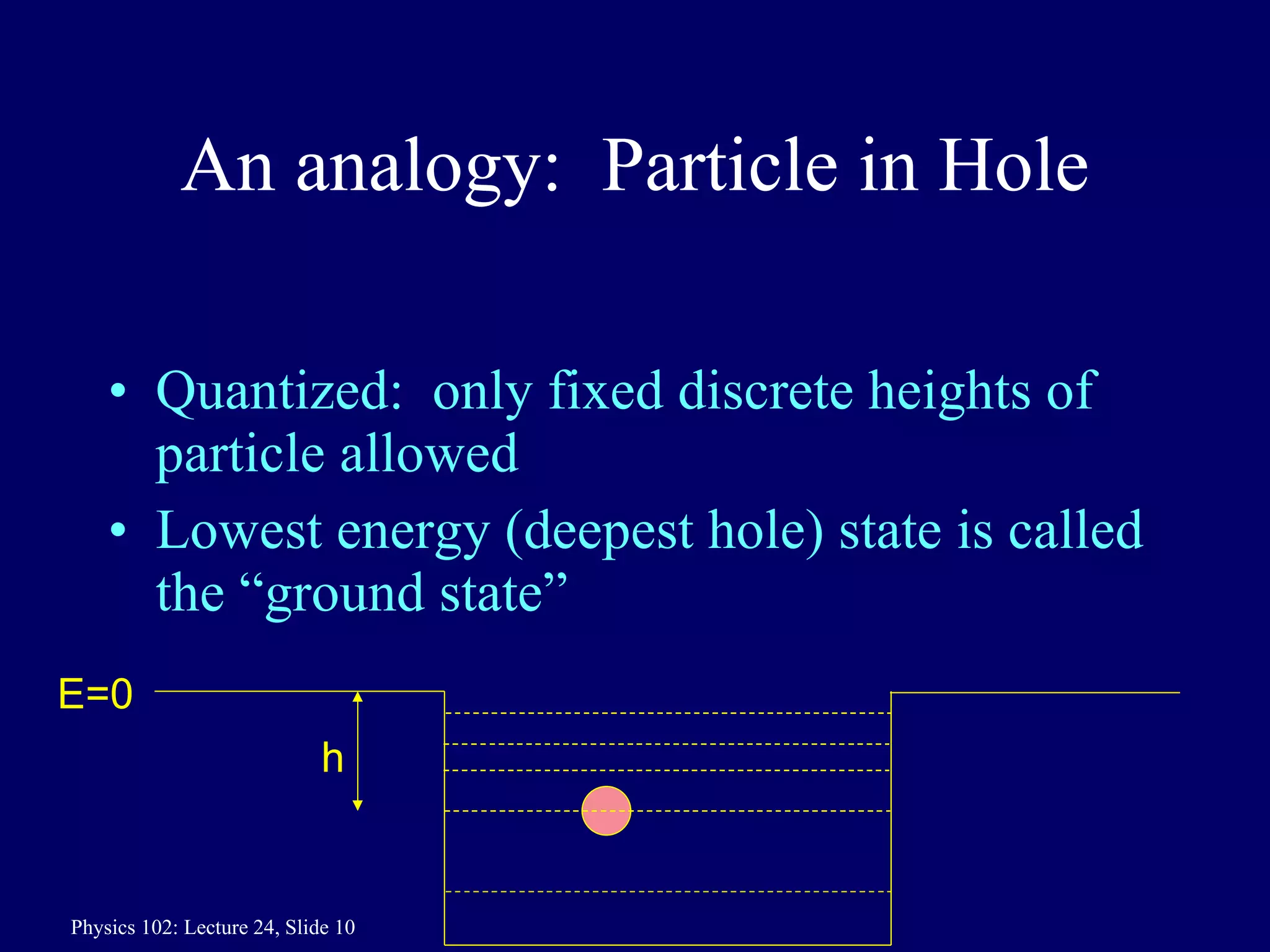
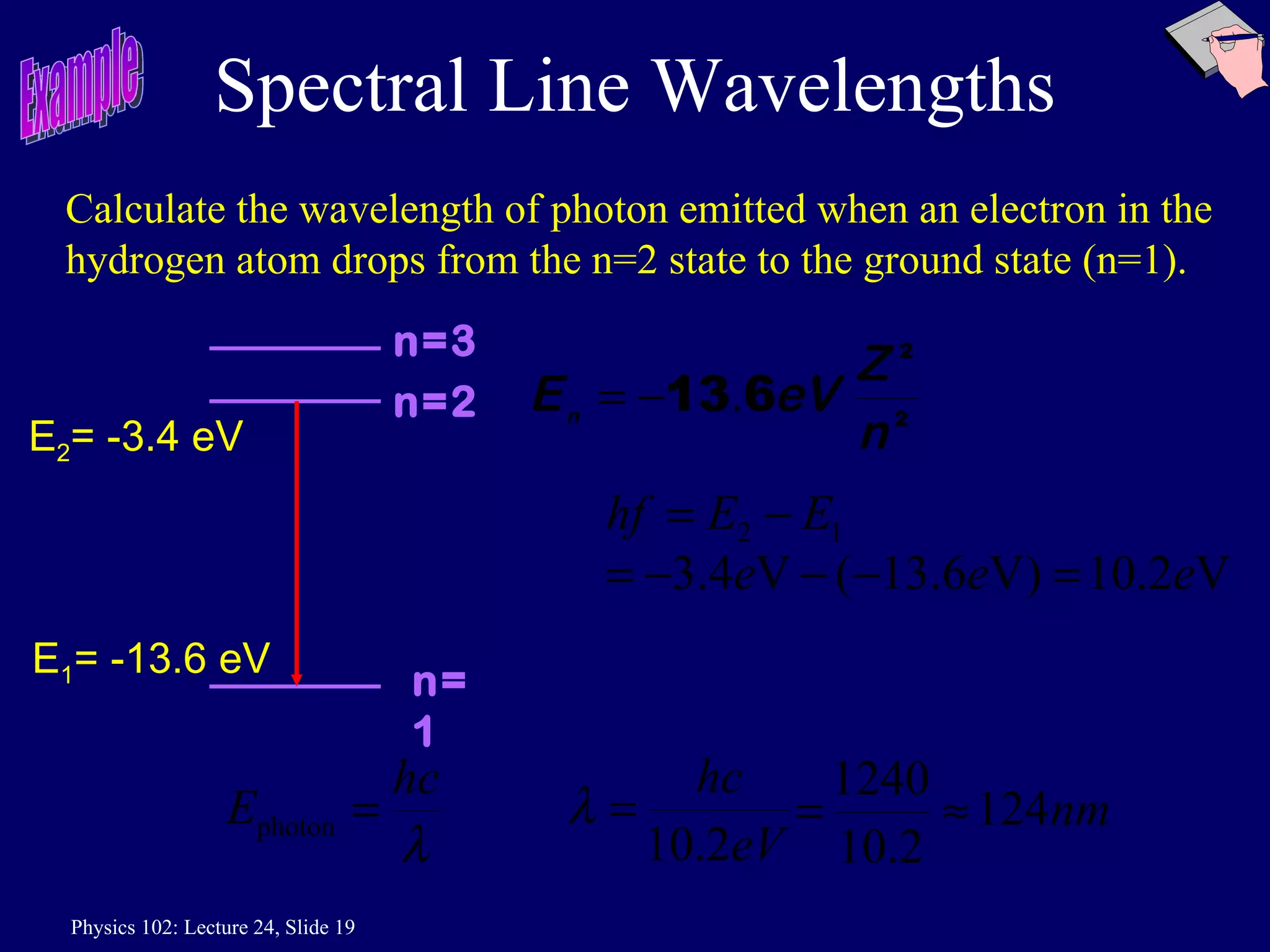
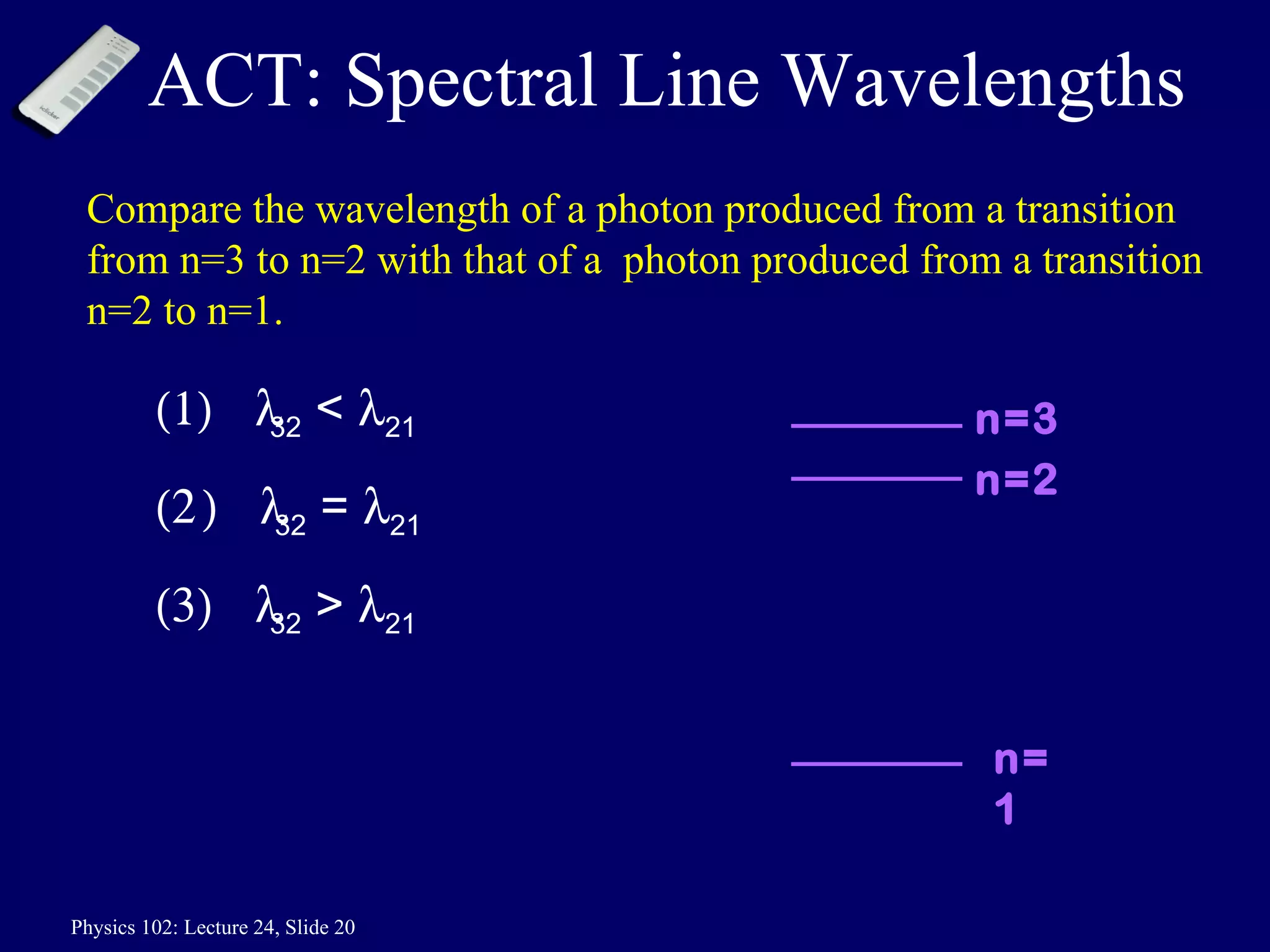
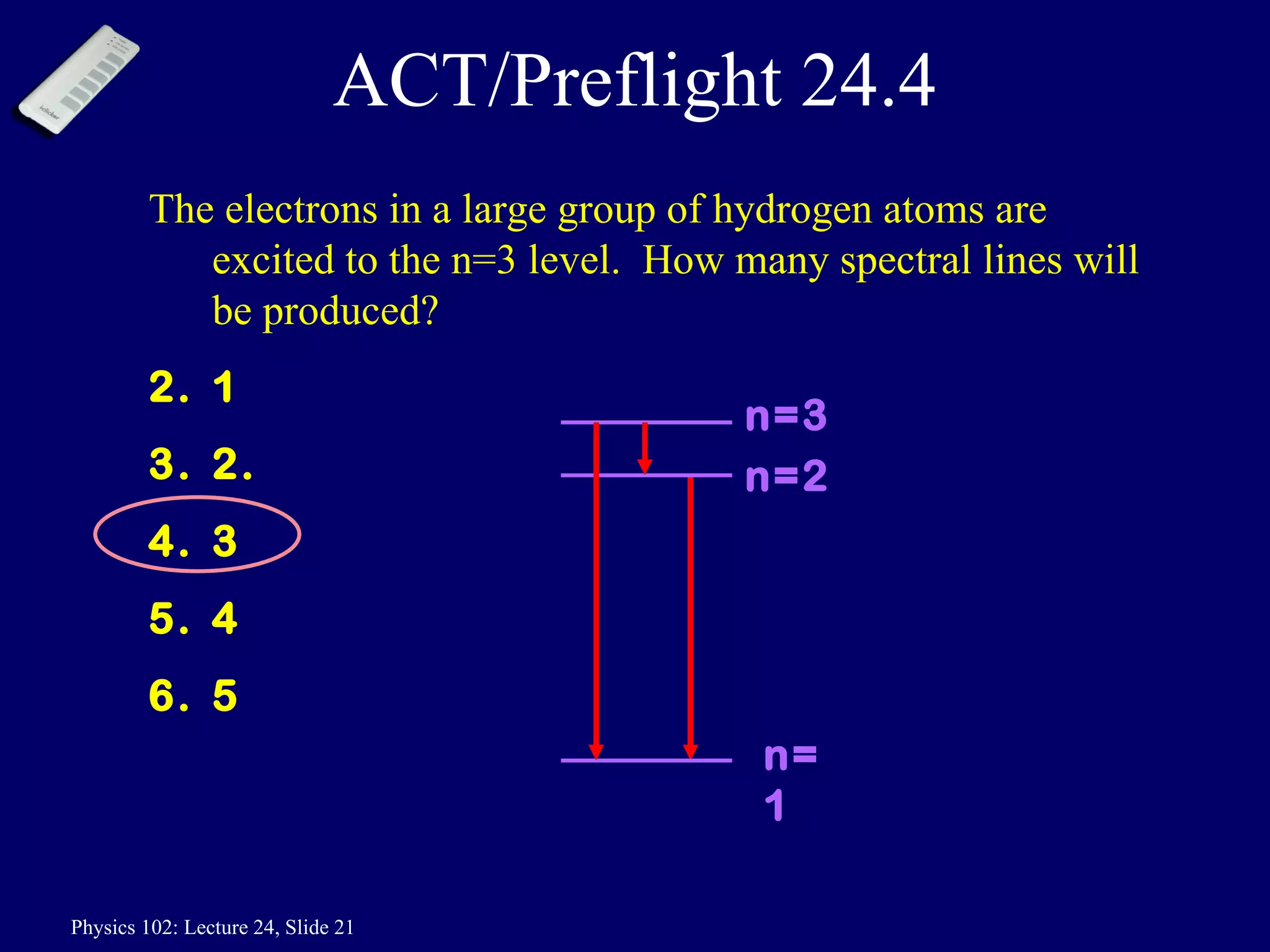
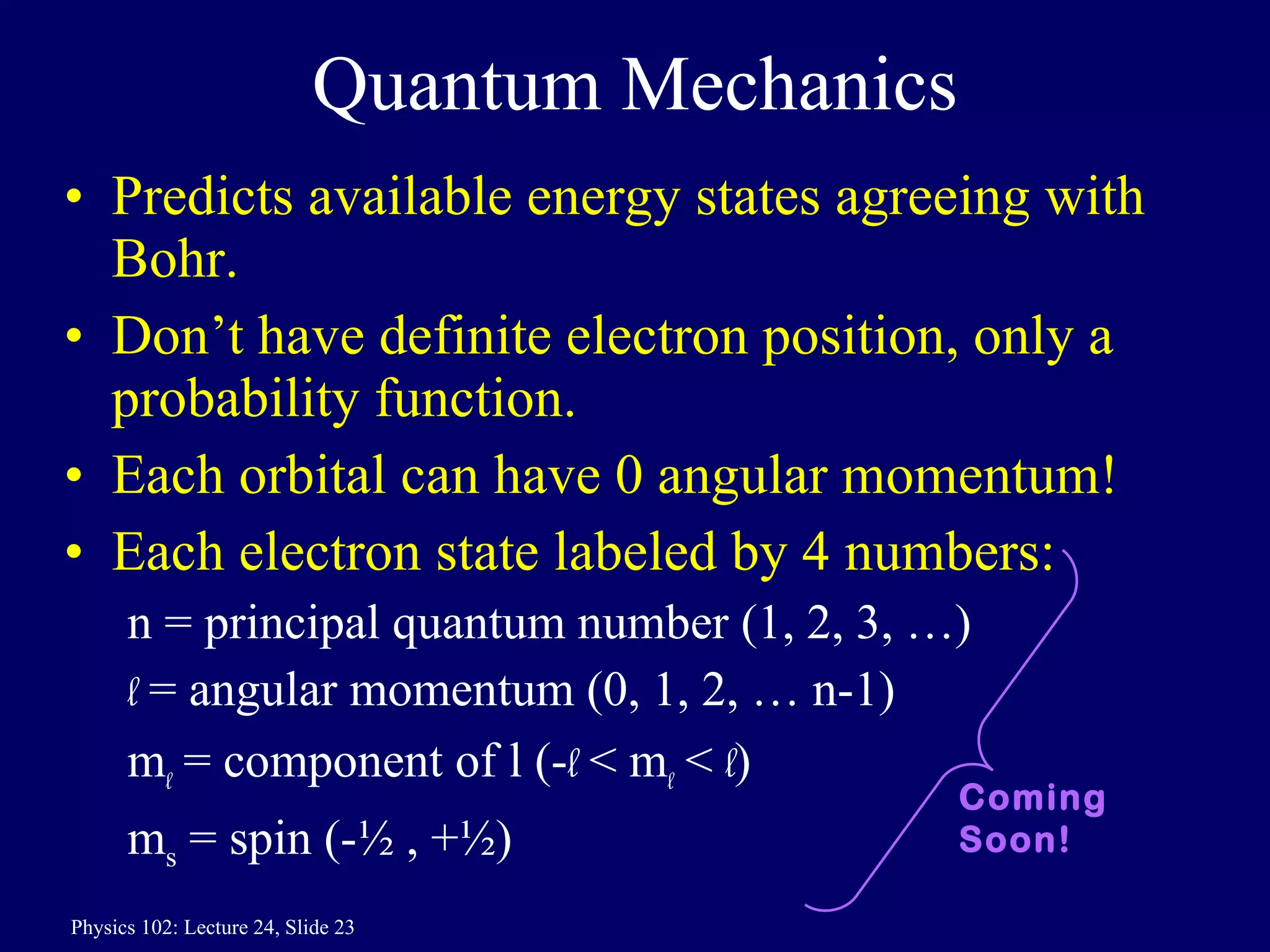
1) The Bohr model of the atom provides accurate predictions of electron energy levels but is not physically realistic, as electrons do not actually orbit the nucleus in defined orbits.
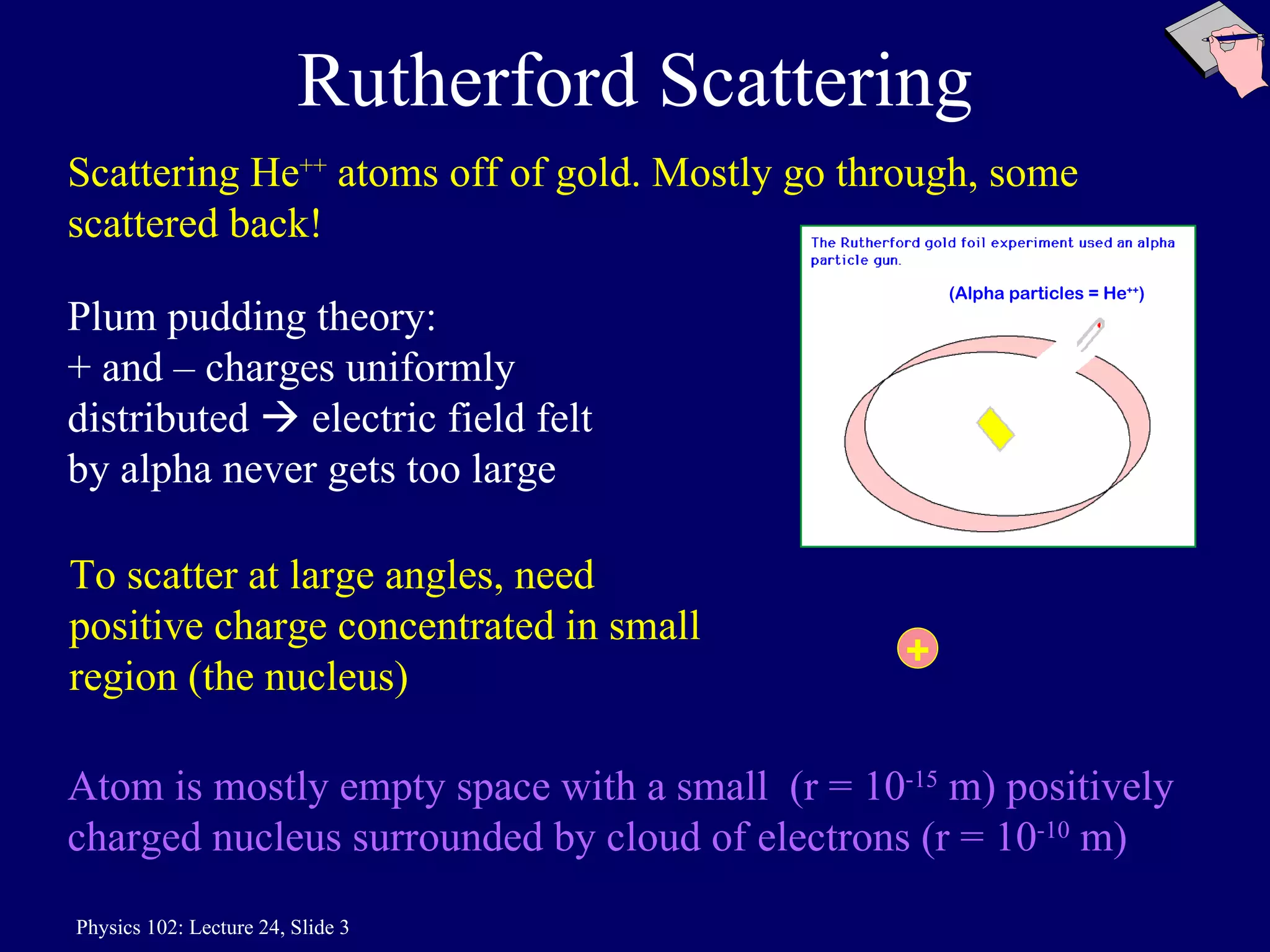
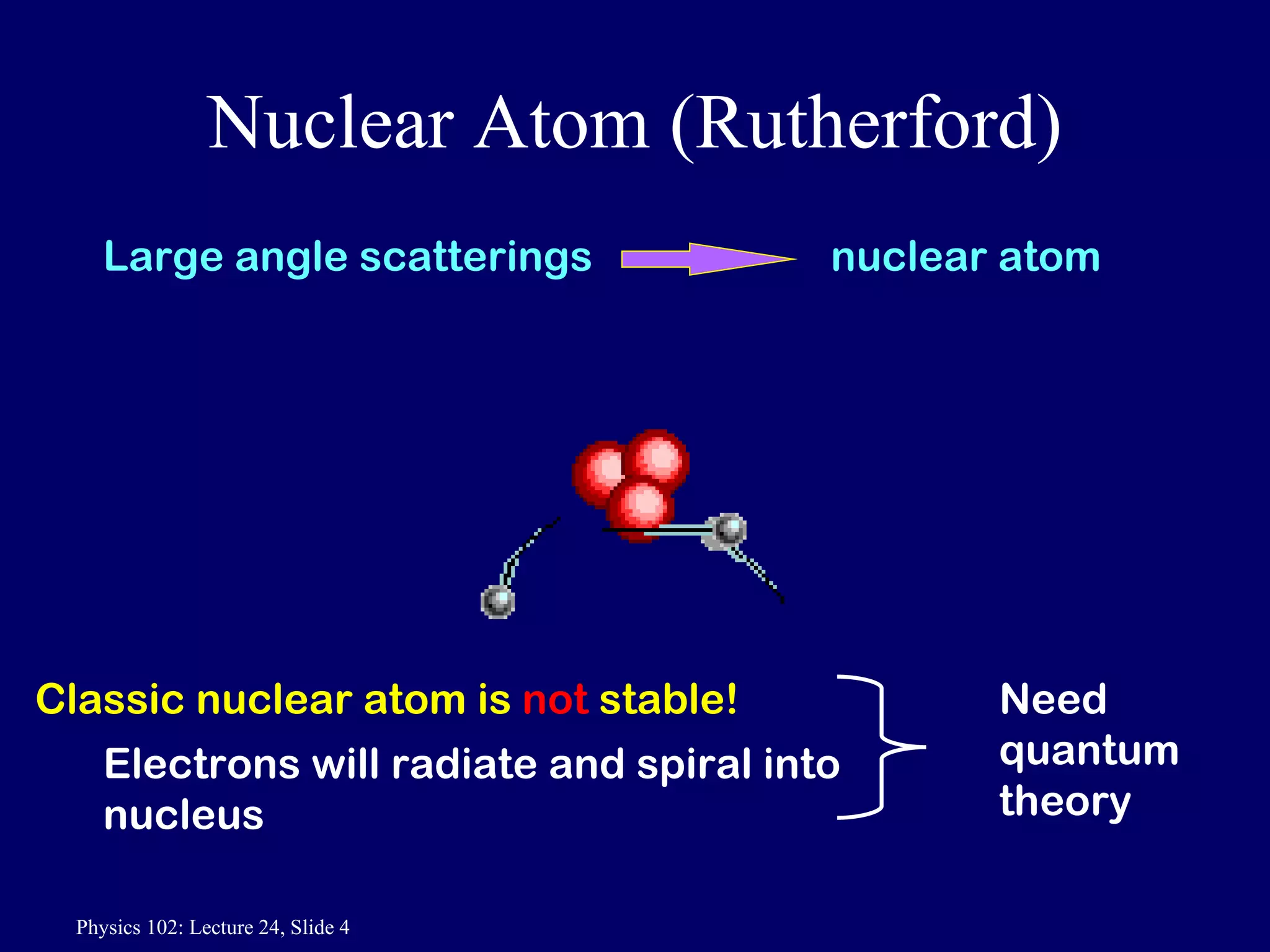
2) Rutherford's nuclear model, based on his gold foil experiment, established that atoms are mostly empty space with a small, dense positively charged nucleus at the center.
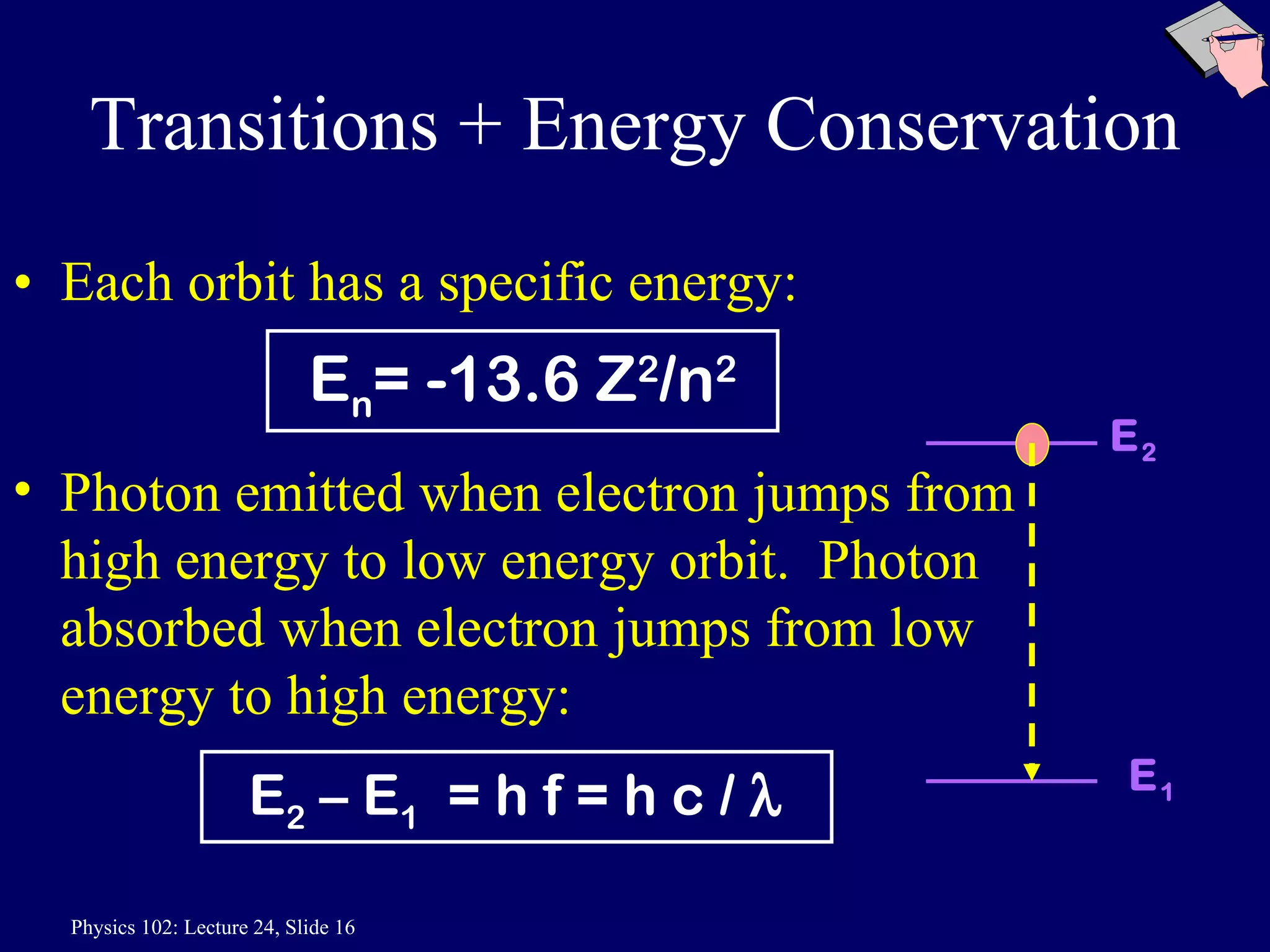
3) Bohr's model incorporated Rutherford's nuclear model and quantized electron orbits, predicting discrete energy levels that explained atomic emission spectra. However, a fully correct quantum mechanical model was still needed.