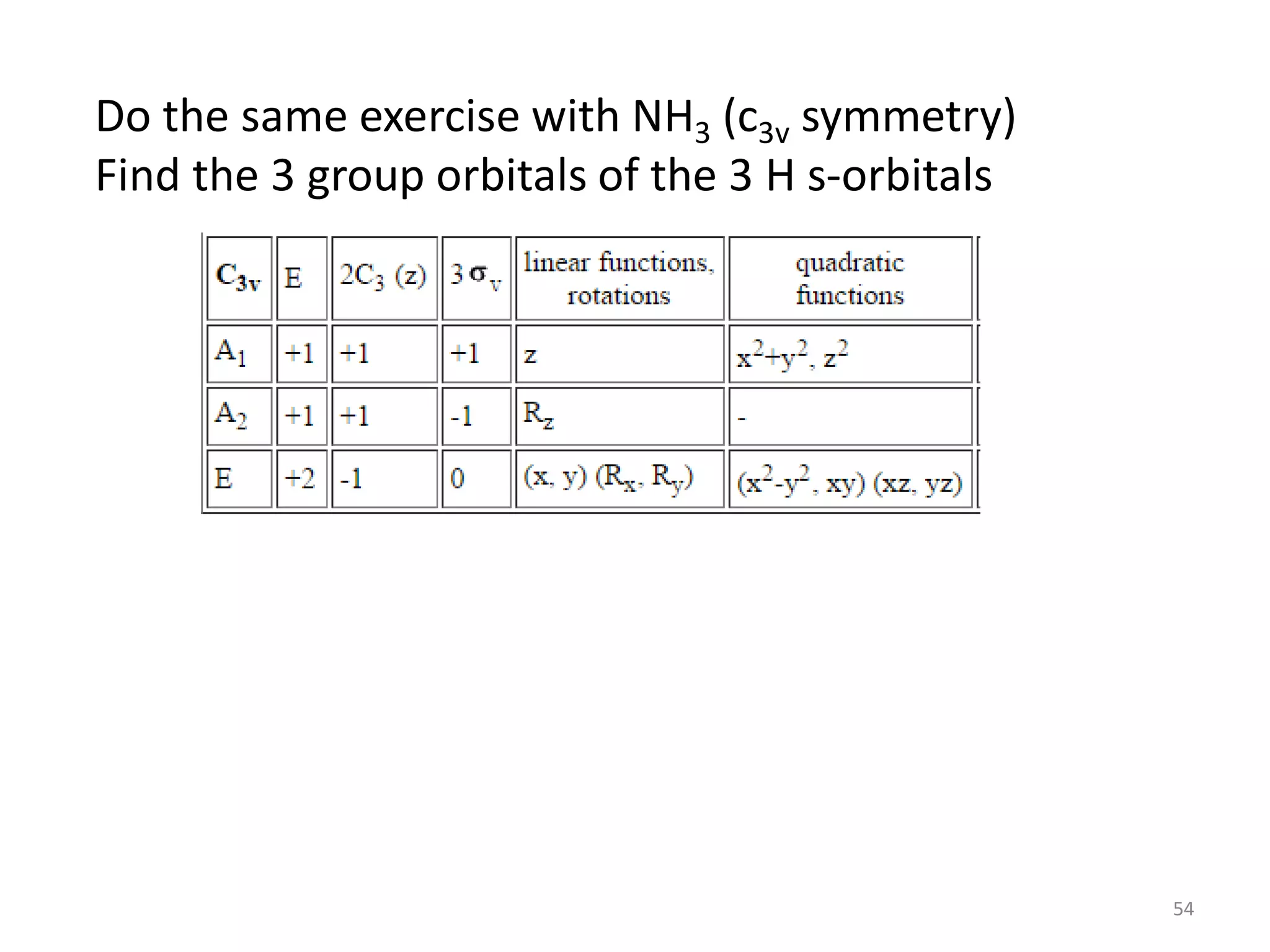
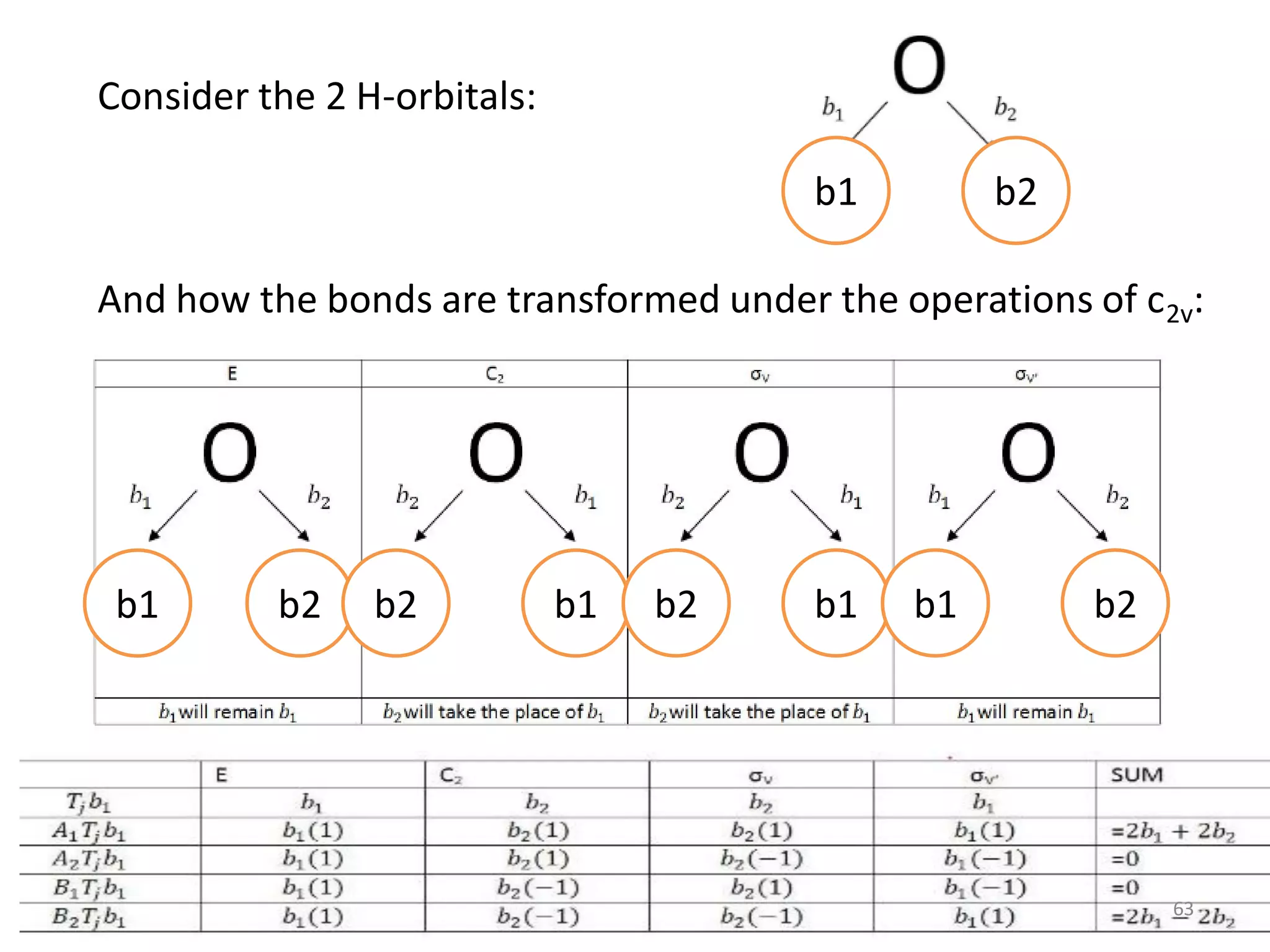
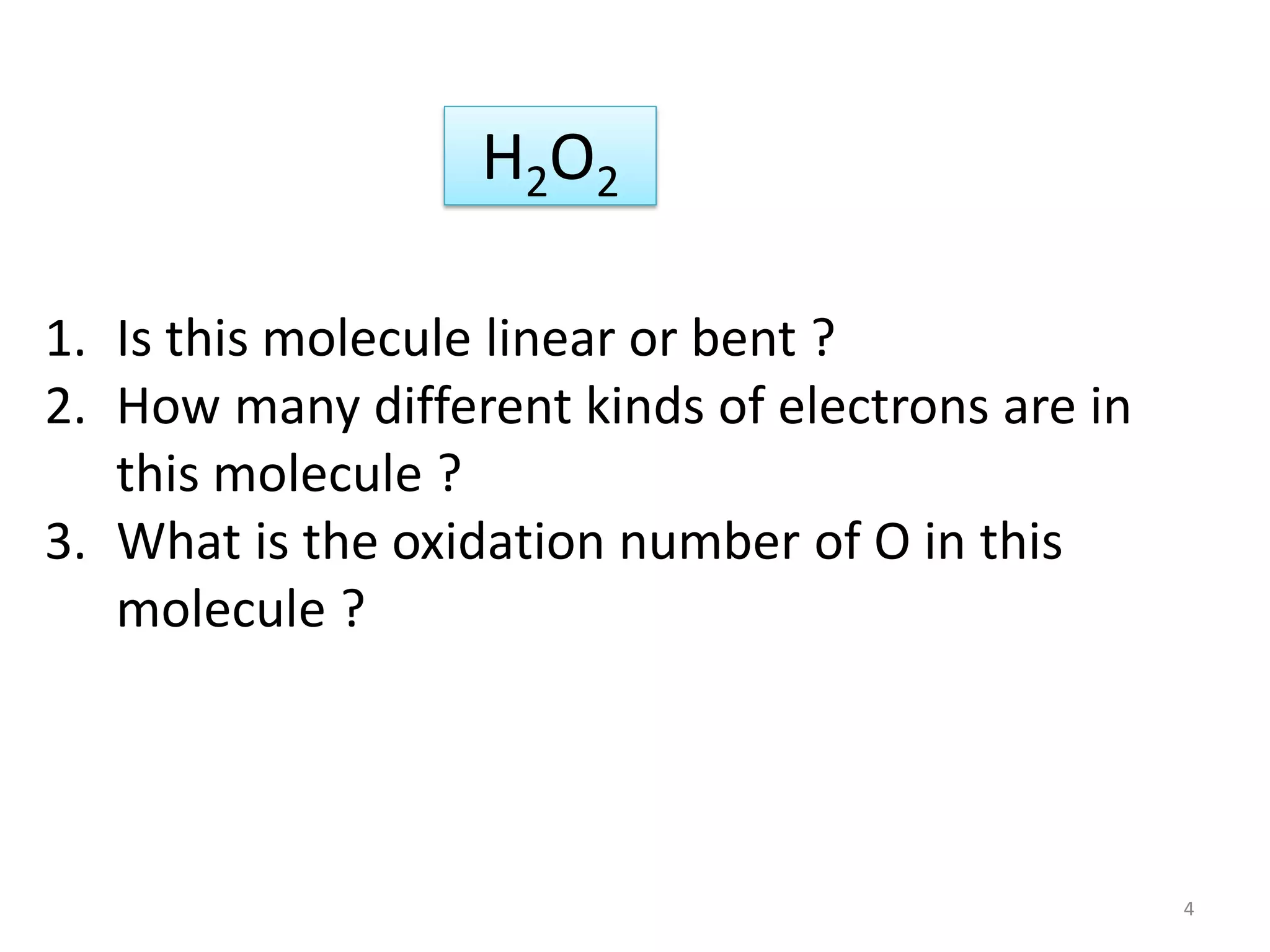
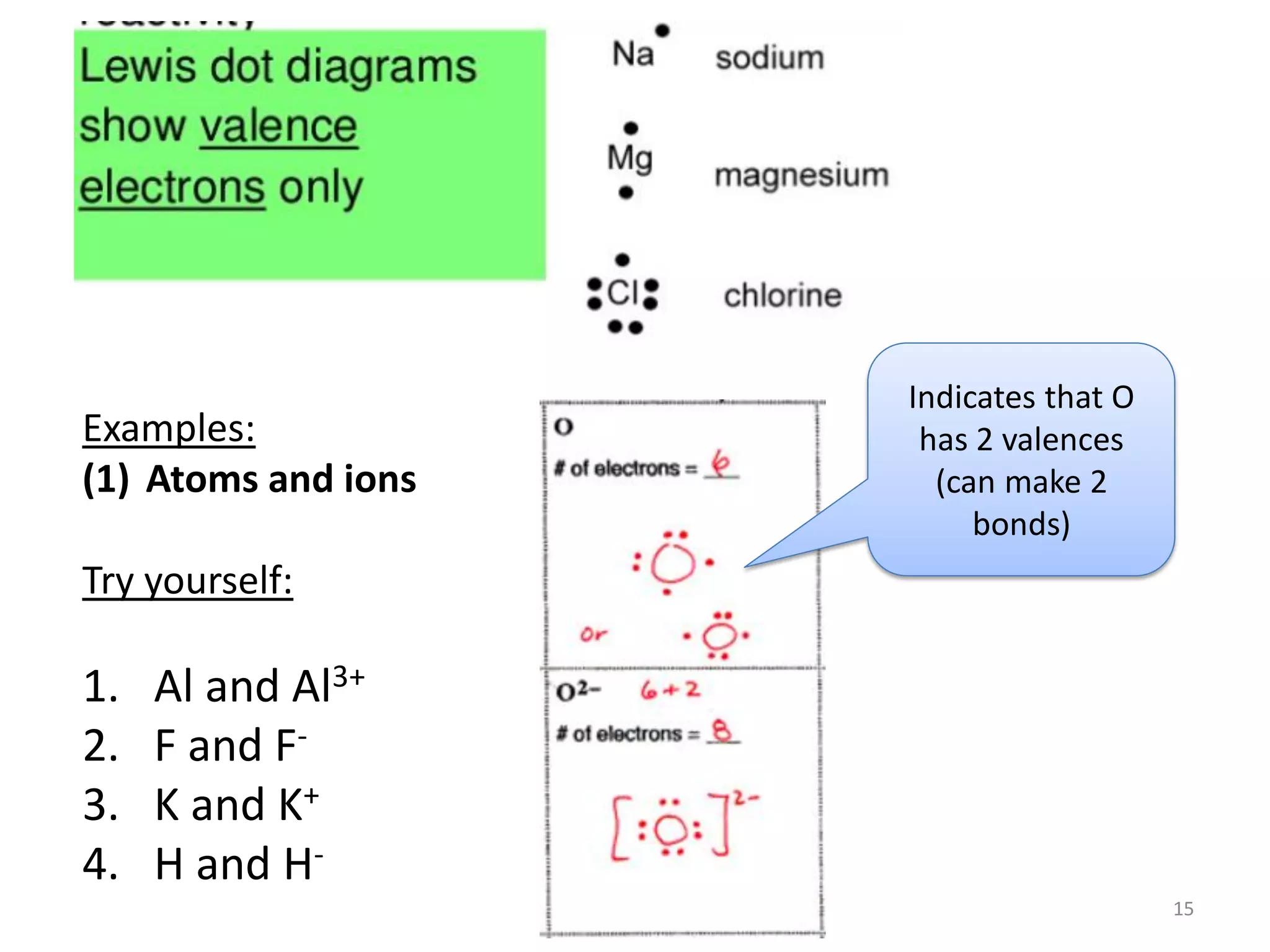
This document covers bonding theories including molecular orbital theory, valence bond theory, and VSEPR theory. It begins with examples of applying concepts like electronegativity, oxidation states, and formal charge to molecules like O3, H2O2, CO, and transition metal compounds. It then discusses valence shell electron pair repulsion theory and how to predict molecular structures. Next, it introduces valence bond theory and hybridization. Molecular orbital theory is covered last, including forming ligand group orbitals, constructing molecular orbitals, and discussing applications to coordination compounds and aromatic ligands.






































![Here we cannot explain 6 ligands around the Ni(2+).
In this case we have to use the “outer” 4d orbitals to form a hybrid:
Use a sp3d2 “outer shell” complex
Explain the bonding in a [Fe(CN)6] 4- complex
45](https://image.slidesharecdn.com/bondingtheories2017-171020114012/75/Bonding-theories-2017-45-2048.jpg)