The document provides an overview of atomic structure, including the discovery and properties of positive rays, Planck's quantum theory, and quantum numbers that describe electron behavior in atoms. It outlines the shapes of orbital and distribution rules for electrons, including Aufbau's principle, Pauli’s exclusion principle, and Hund’s rule. Additionally, the document includes a series of multiple-choice questions to test understanding of these concepts.



![Quantum numbers: The Set of numbers that describe the complete
behavior of an electron in an atom [2,1,–1, +1/2]
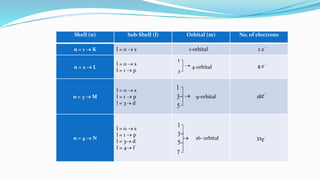
Principle Quantum number: A non-zero, positive integer that describe the
number of shell size of atom and energy of electron in an atom. It is
denoted by ‘n’, n = 1, 2,3 …… also called as K, L, M and N shell
Azimuthal quantum number: it describe the number of sub shell in a shell
also describe the shapes of sub shell represented by ‘l’, l = n – 1
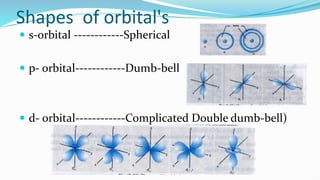
n = 1 l = 0 s-subshell sharp (spherical shape)
n = 2 l = 1 p-subshell principle (dumbbell shape)
n = 3 l = 2 d-subshell diffused (sausage shape)
n = 4 l = 3 f-subshell fundamental (complicated shape)
NOTE: Finely divided spectral lines seen by spectrometer in Bohr’s spectrum
give him about an other energy level that is explained by this quantum number](https://image.slidesharecdn.com/unit-2atomicstructure-240502053138-39b8a33d/85/UNIT-No-2-Atomic-Structure-of-atoms-pptx-5-320.jpg)