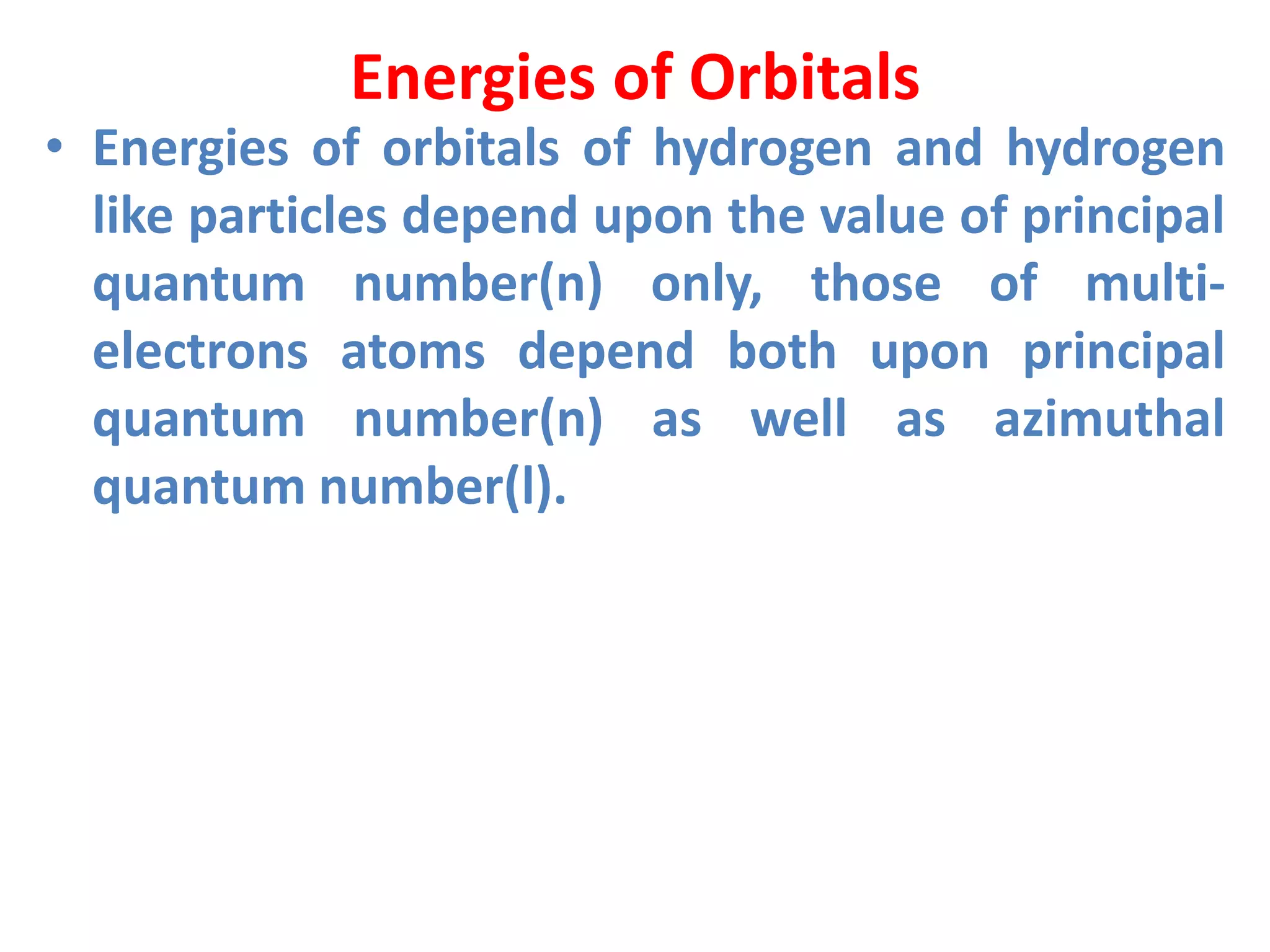
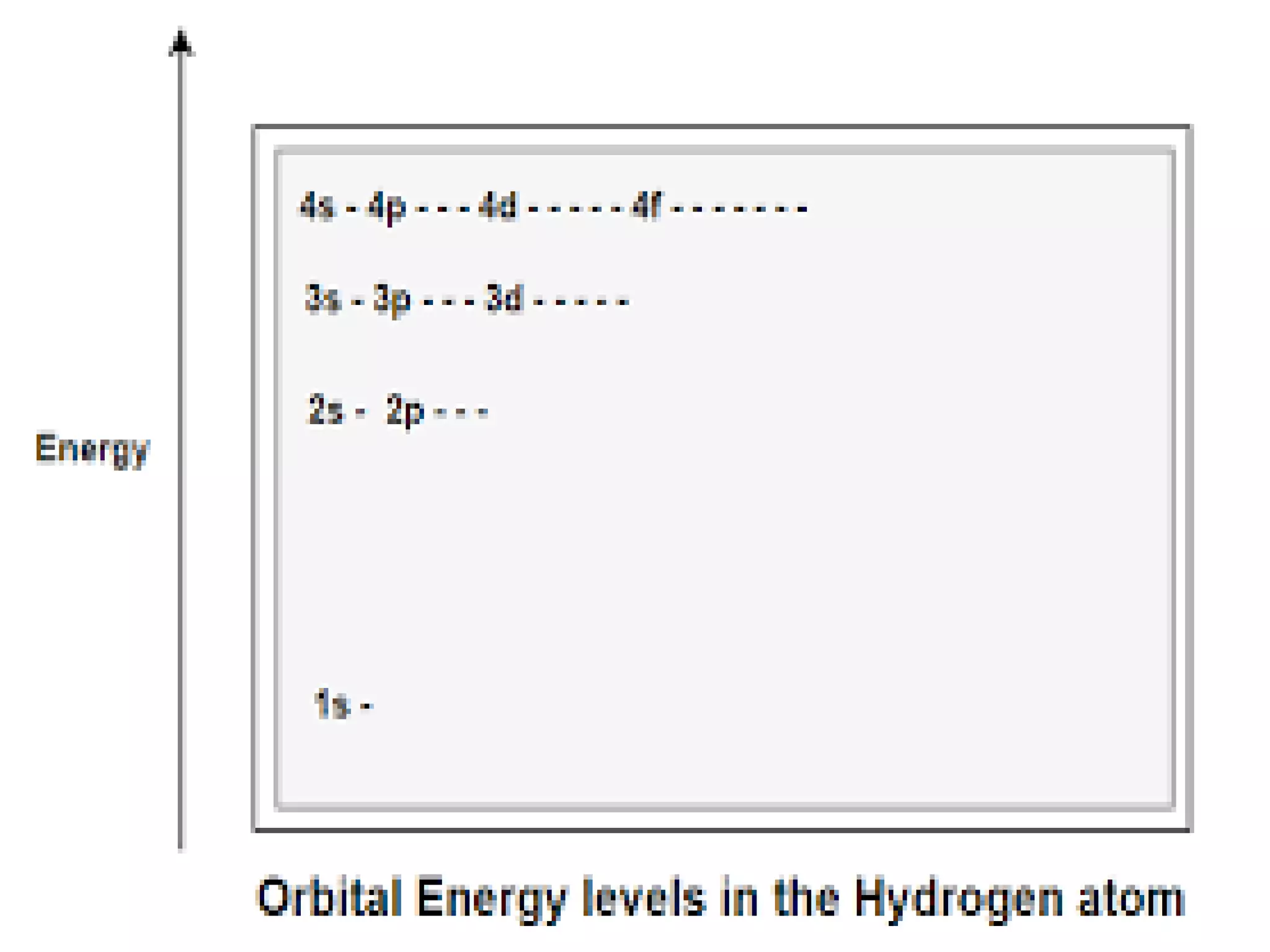
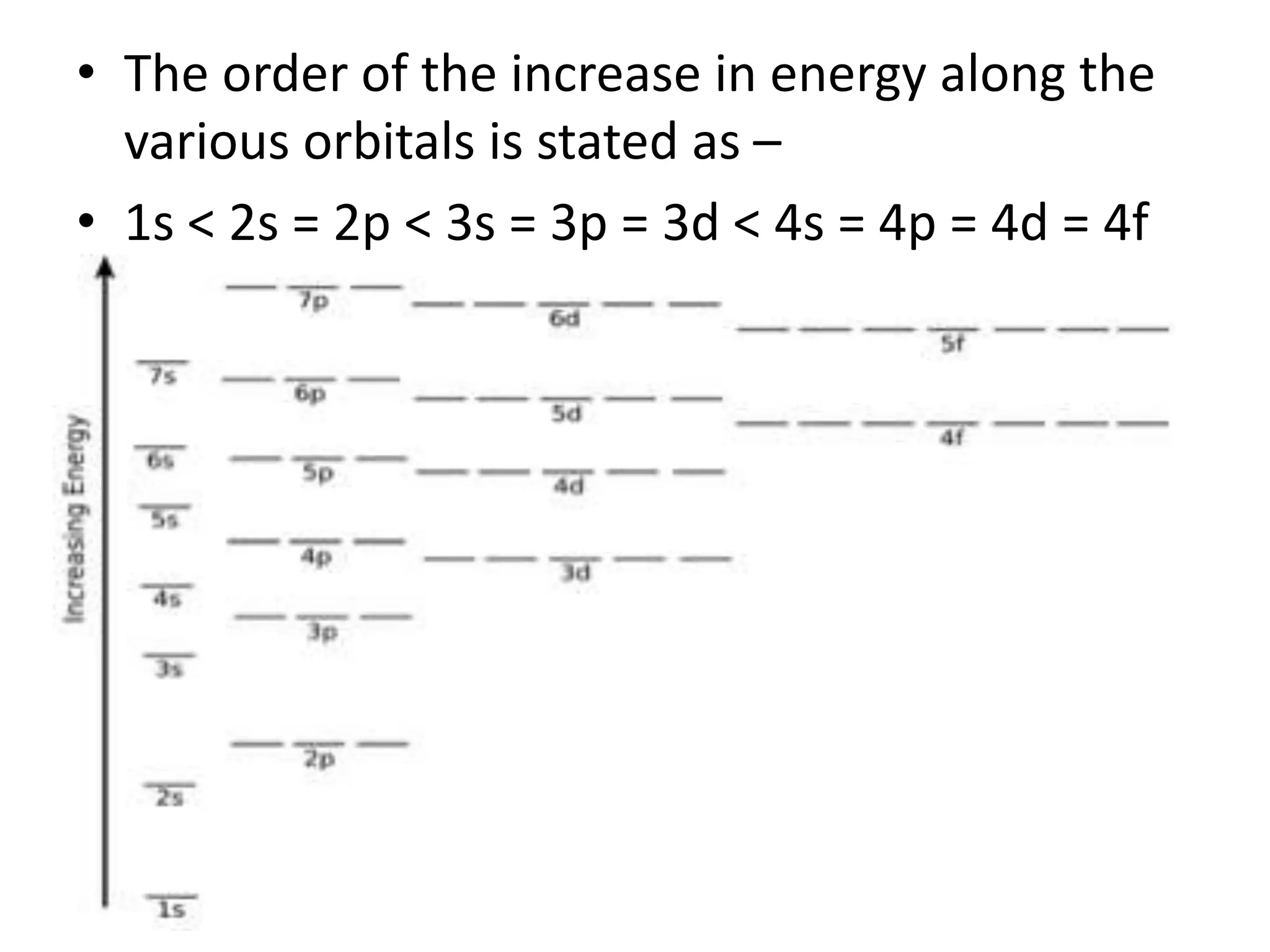
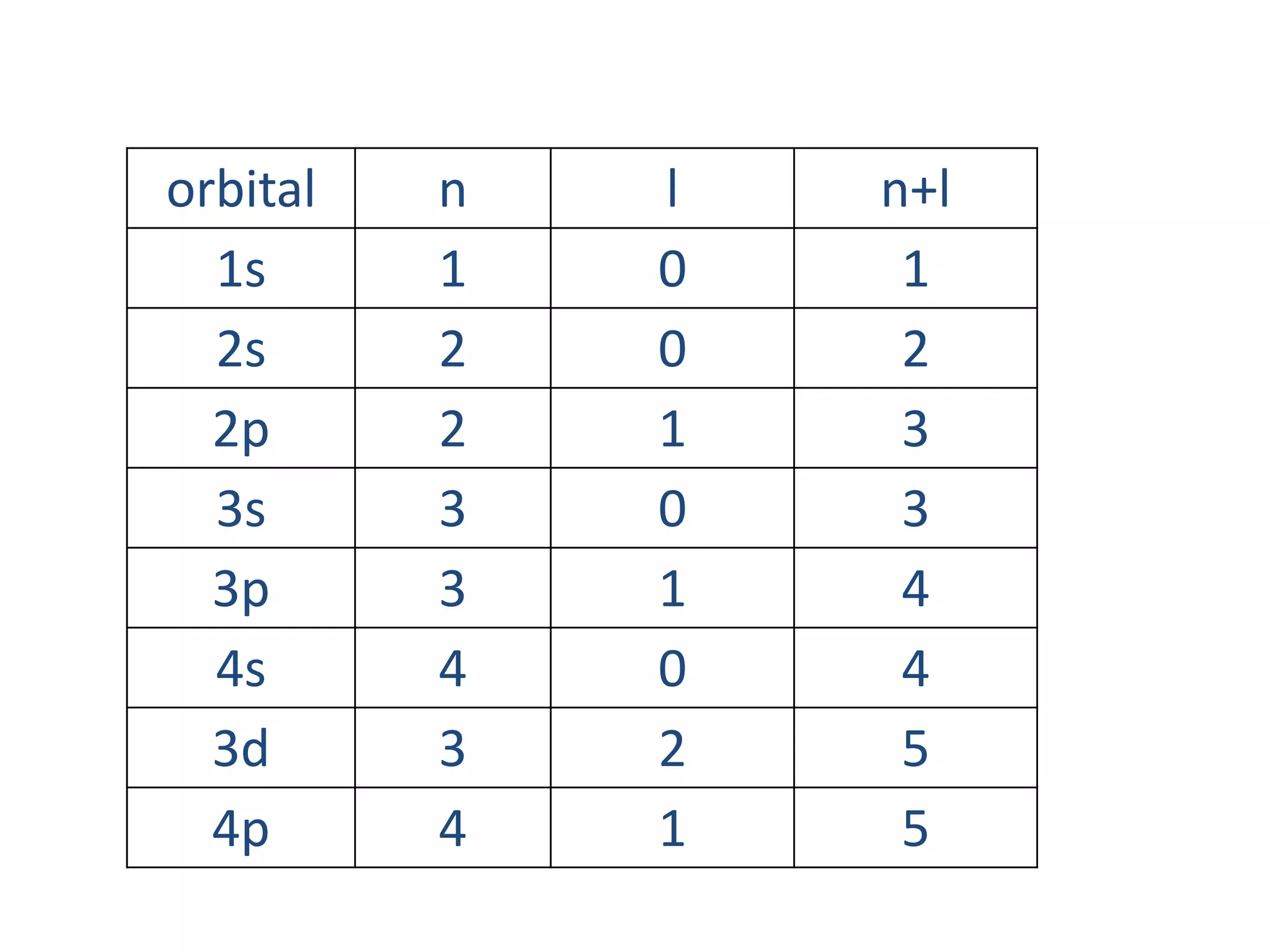
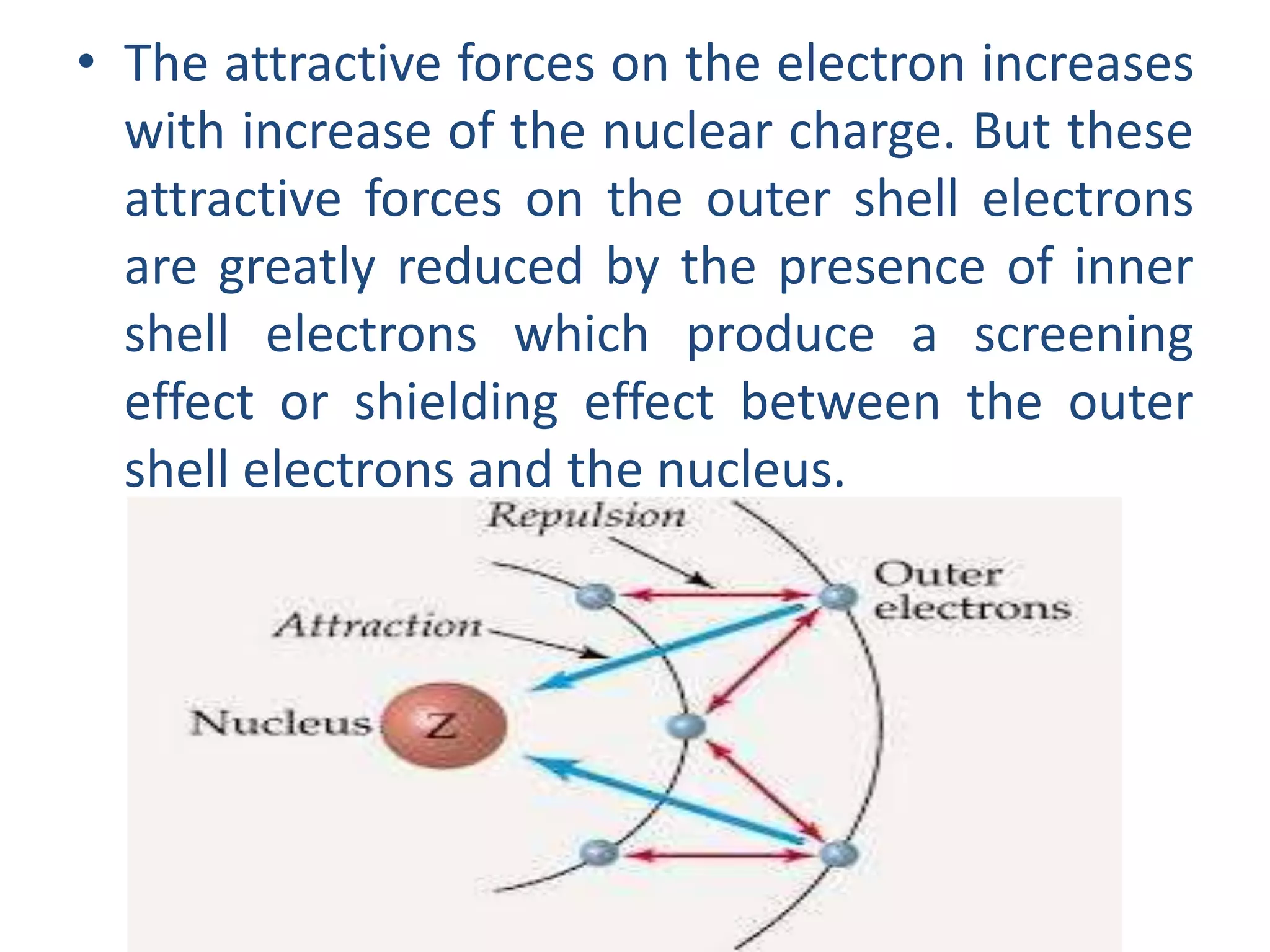
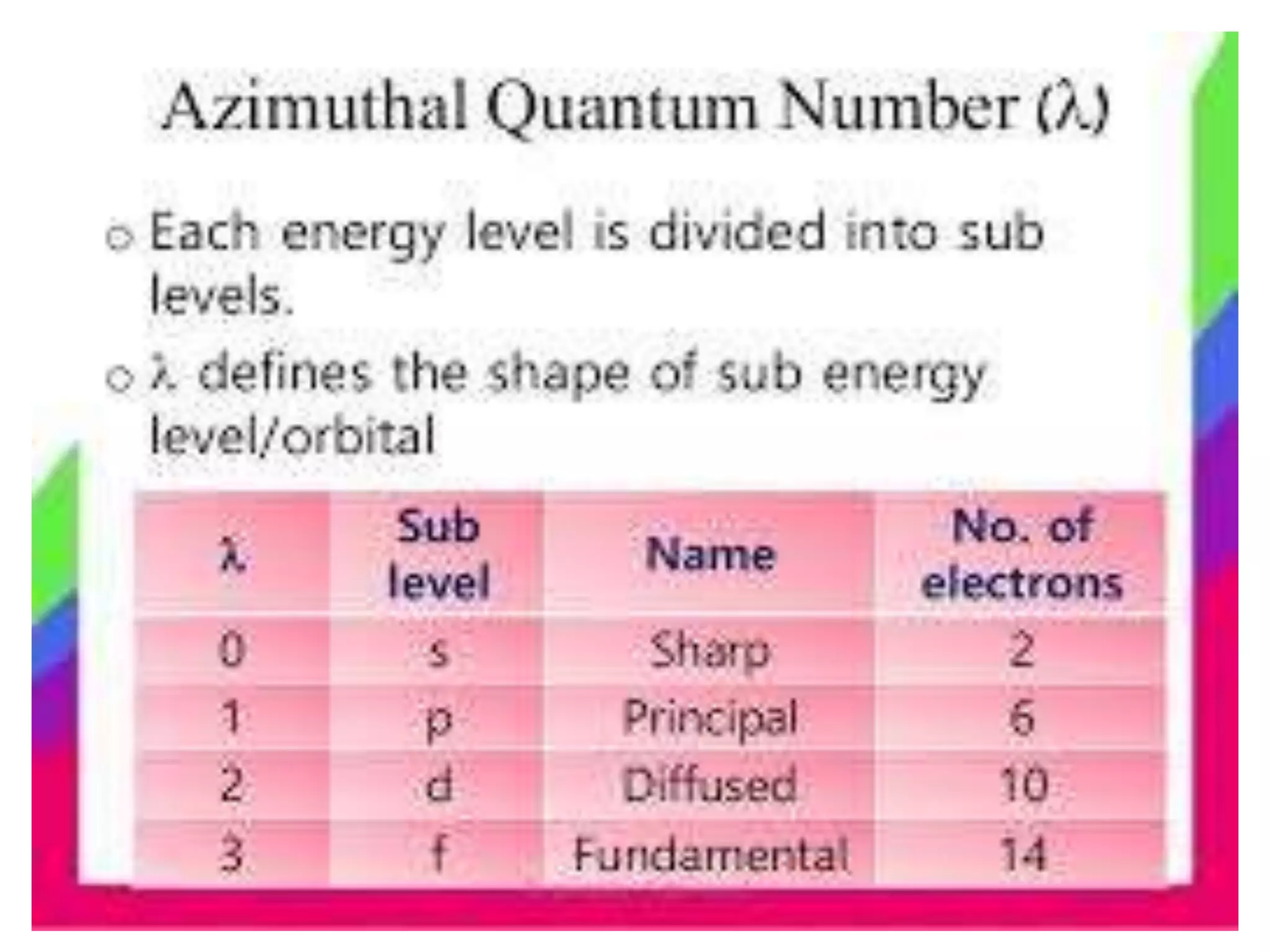
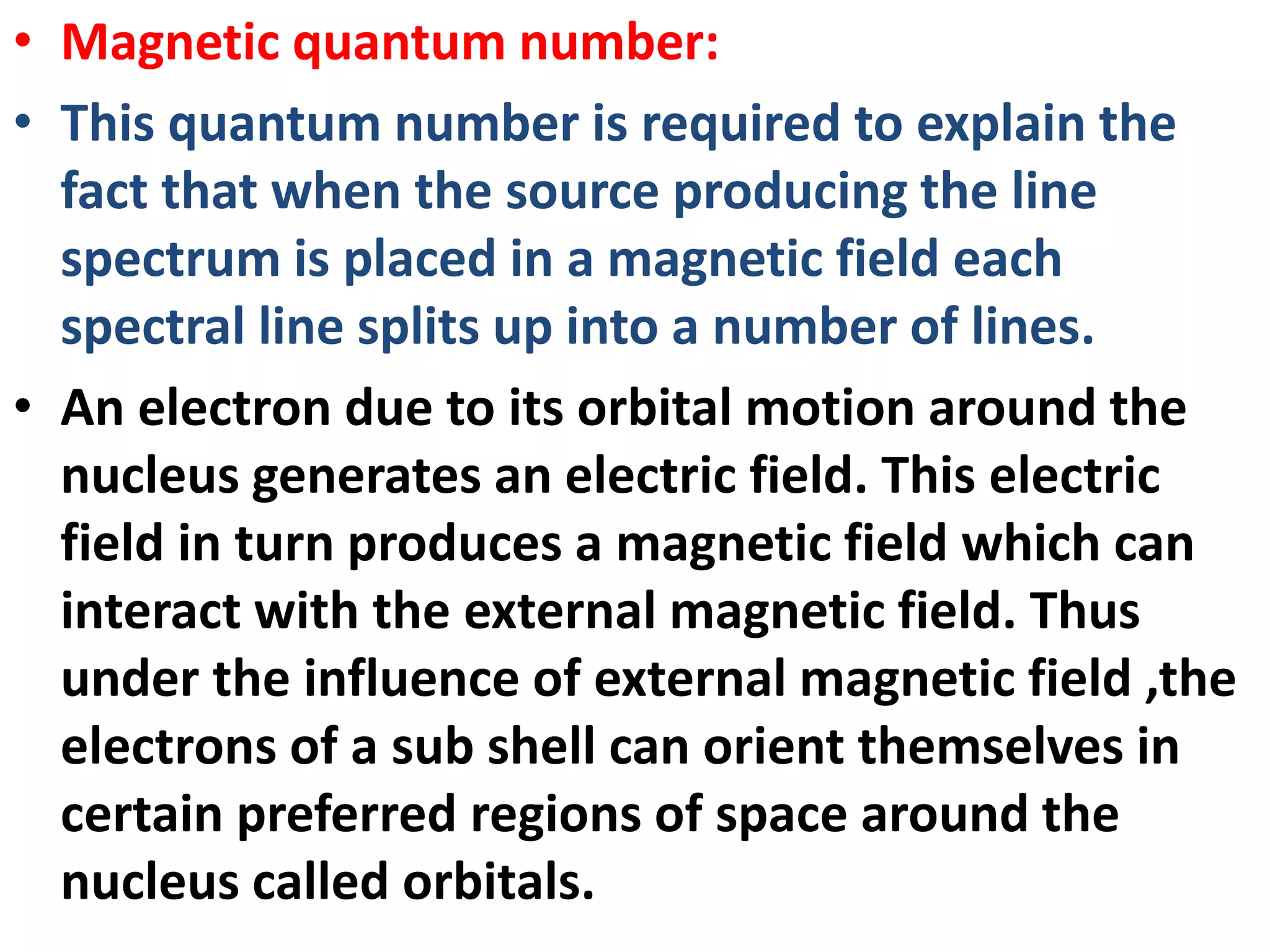
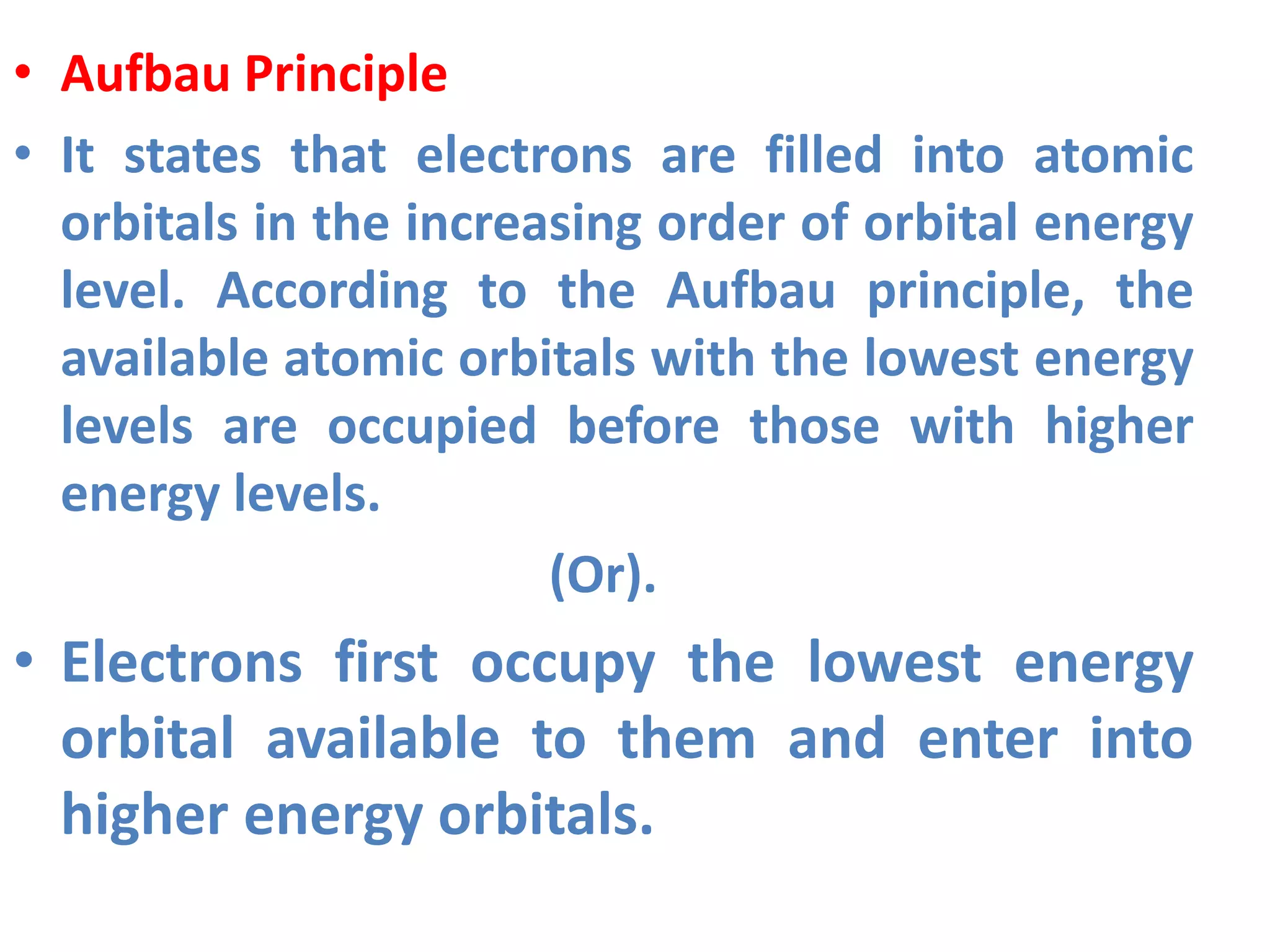
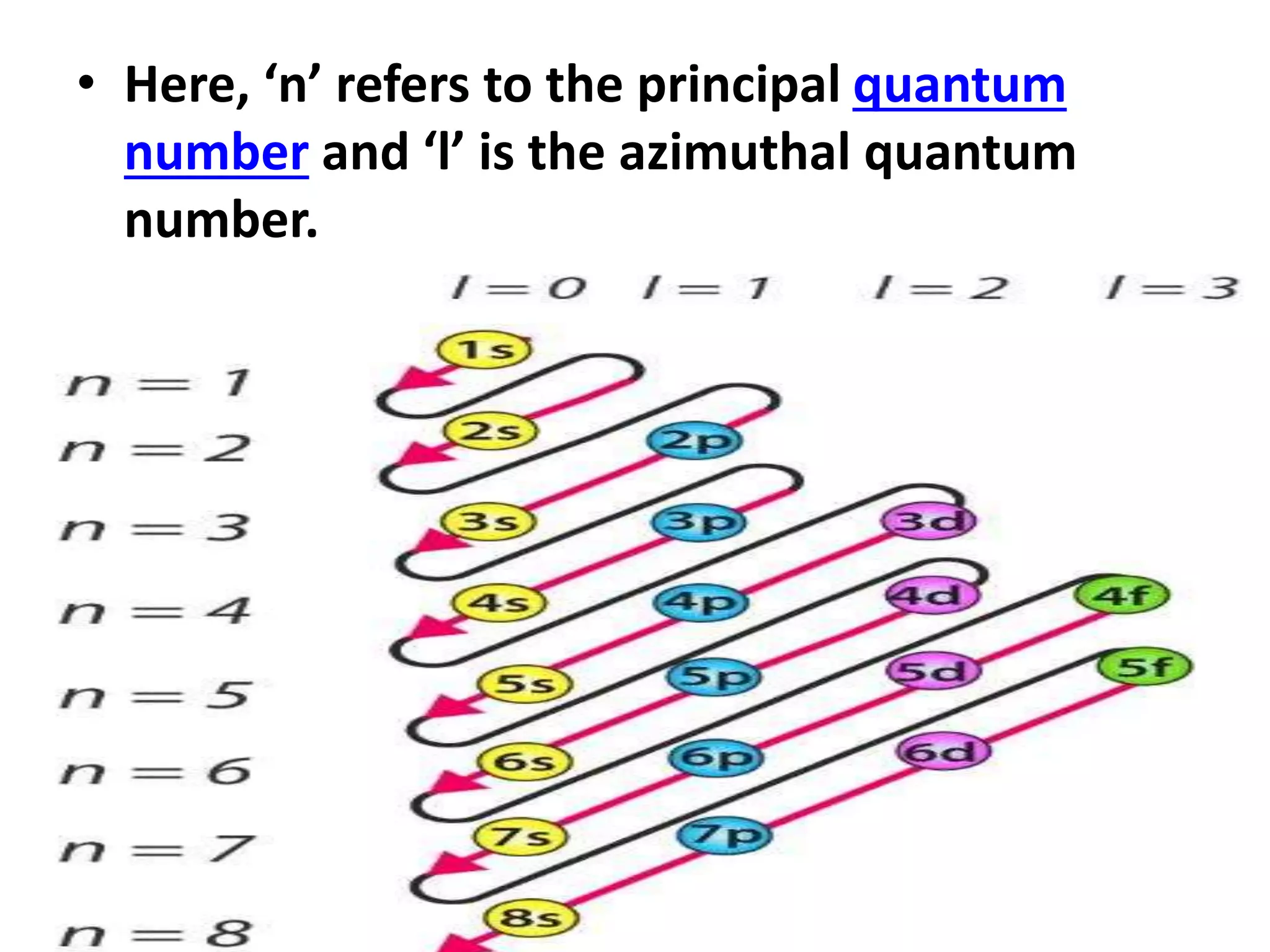
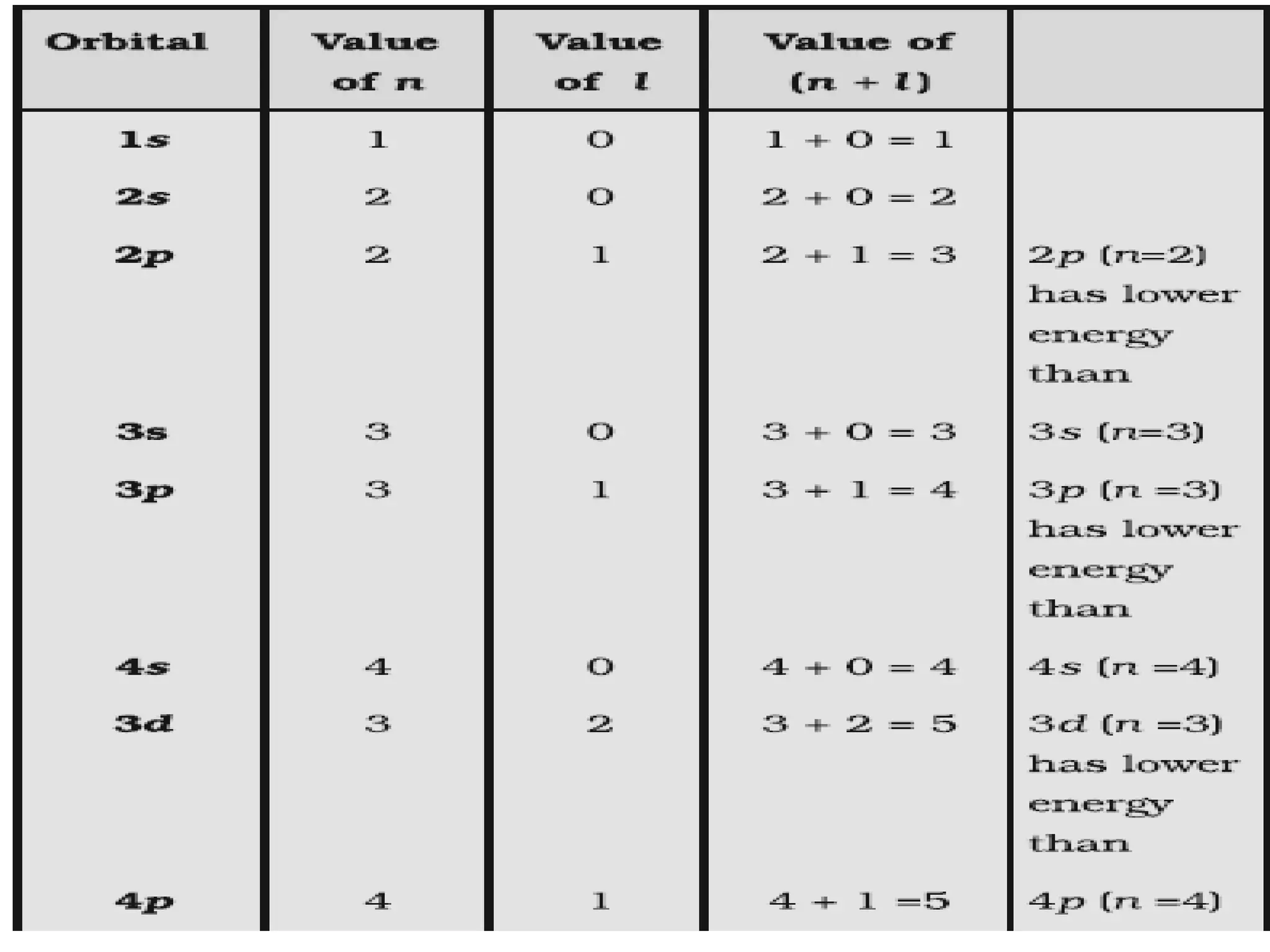
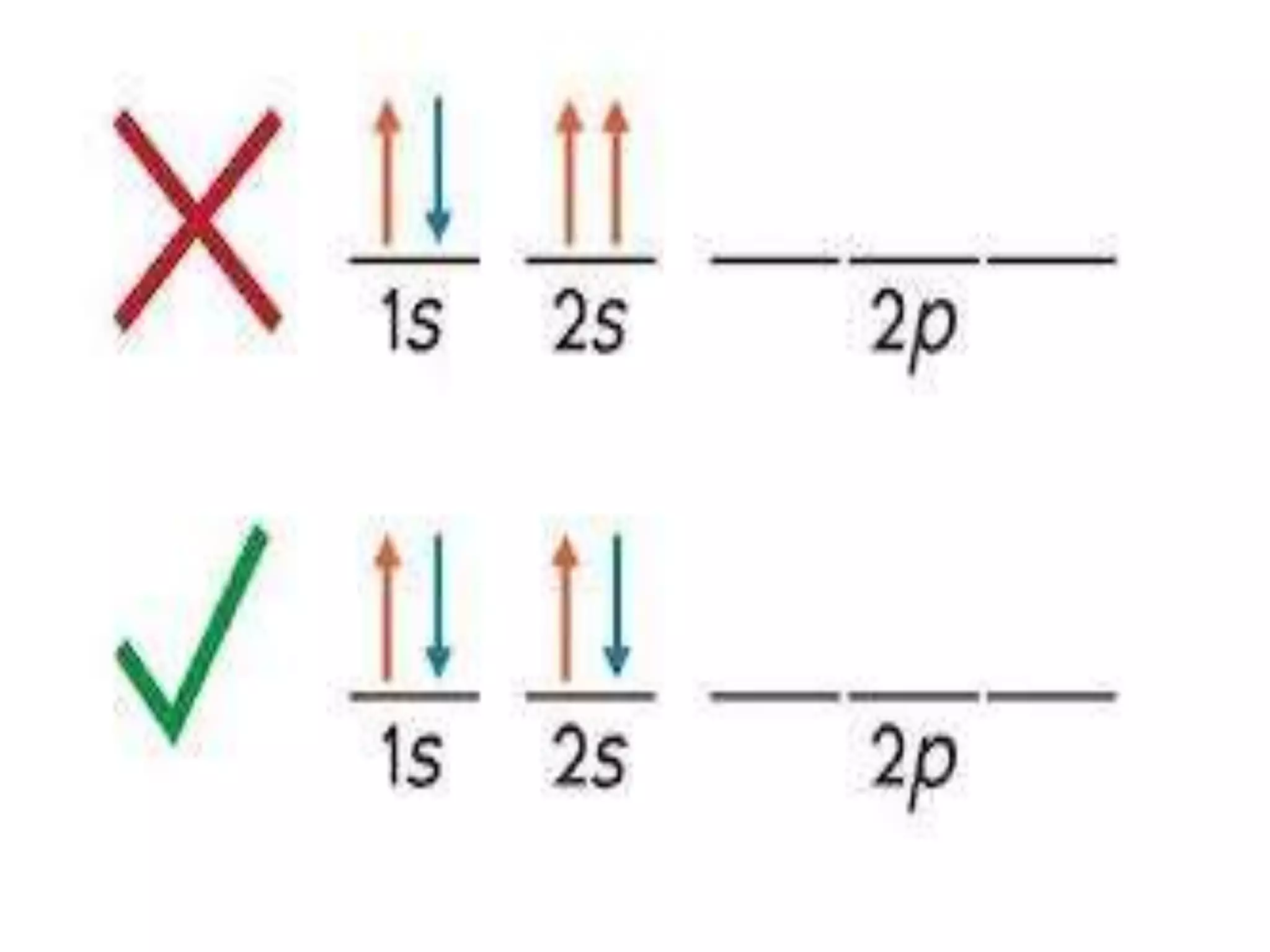
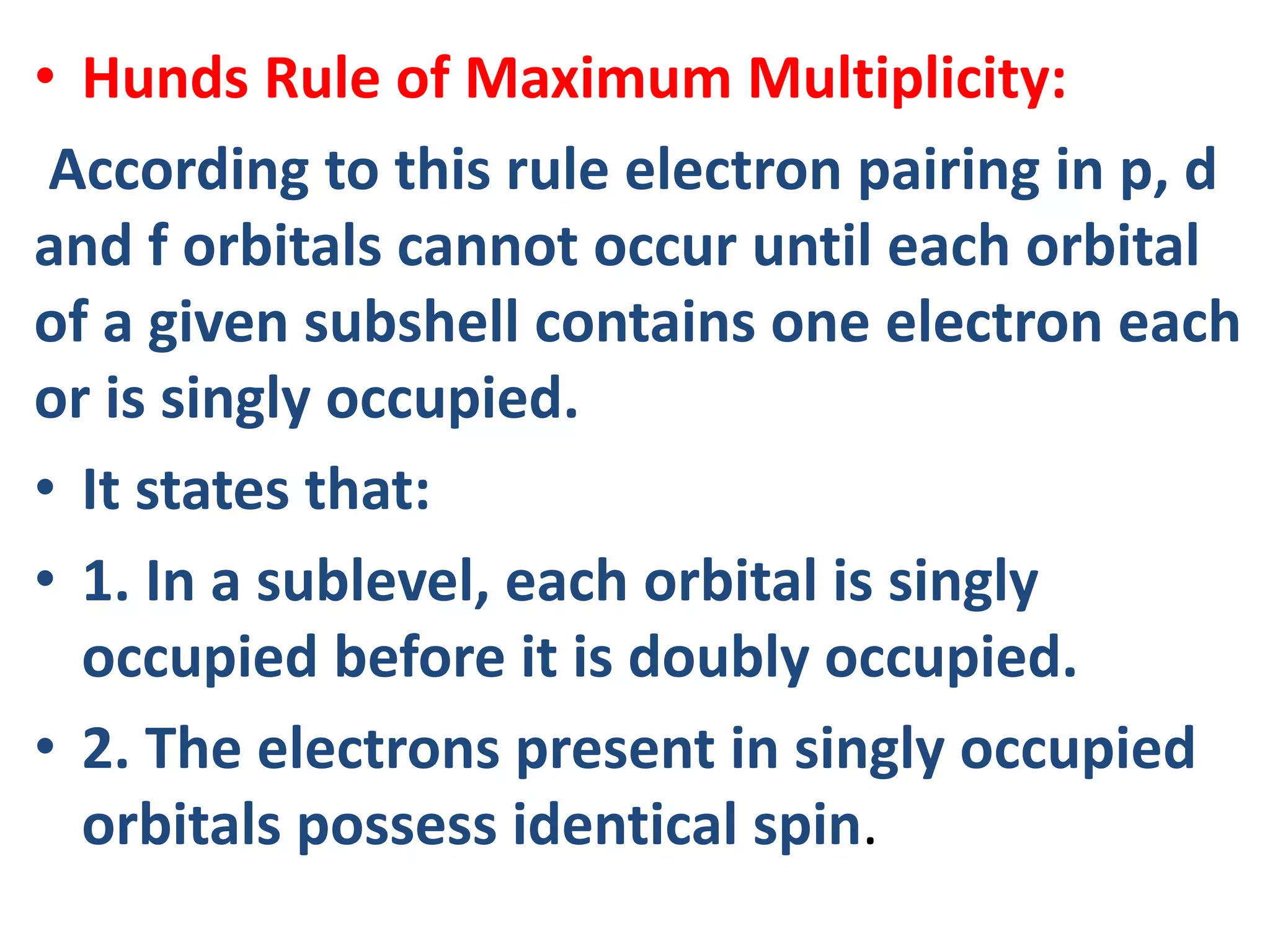
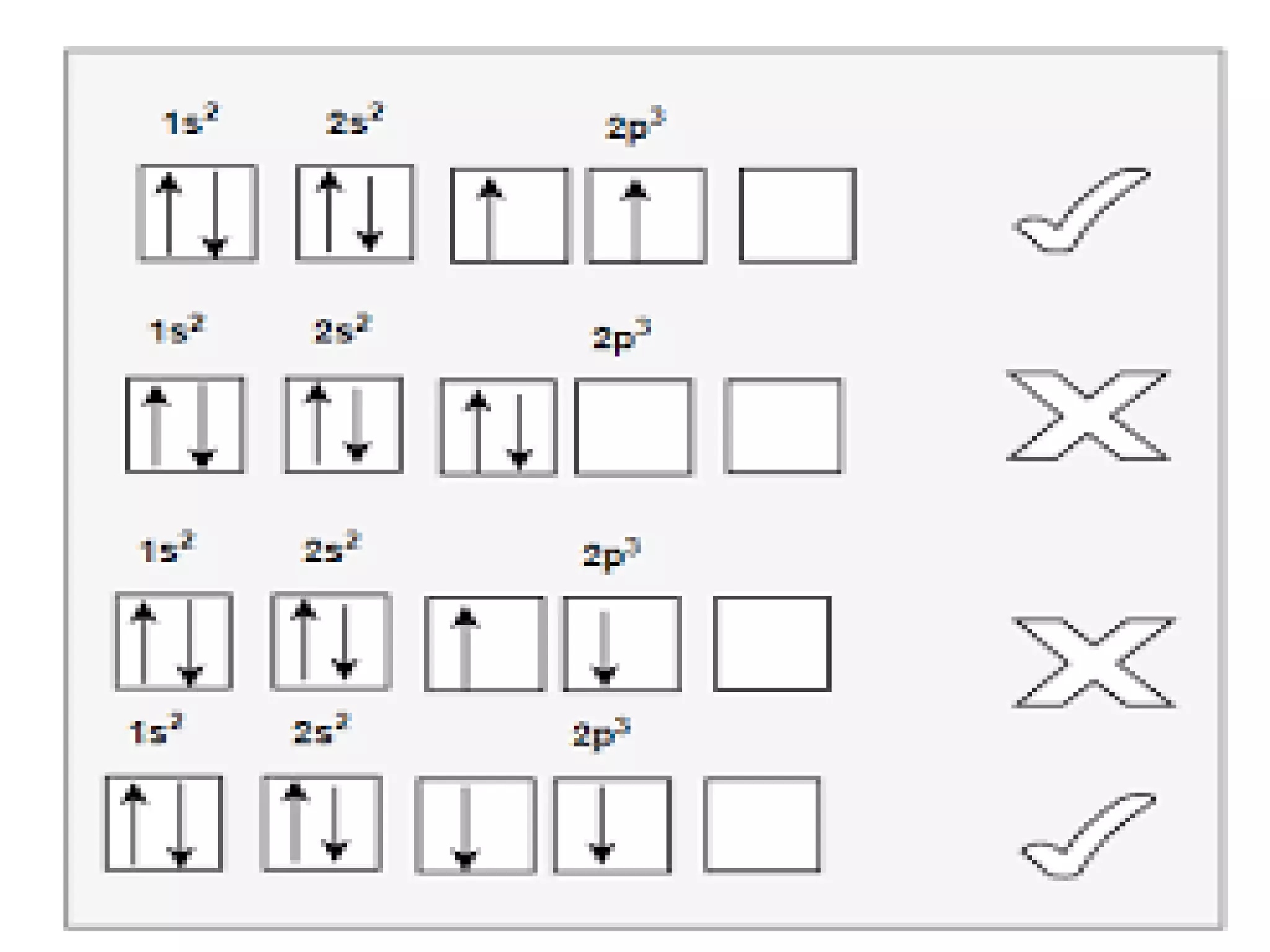
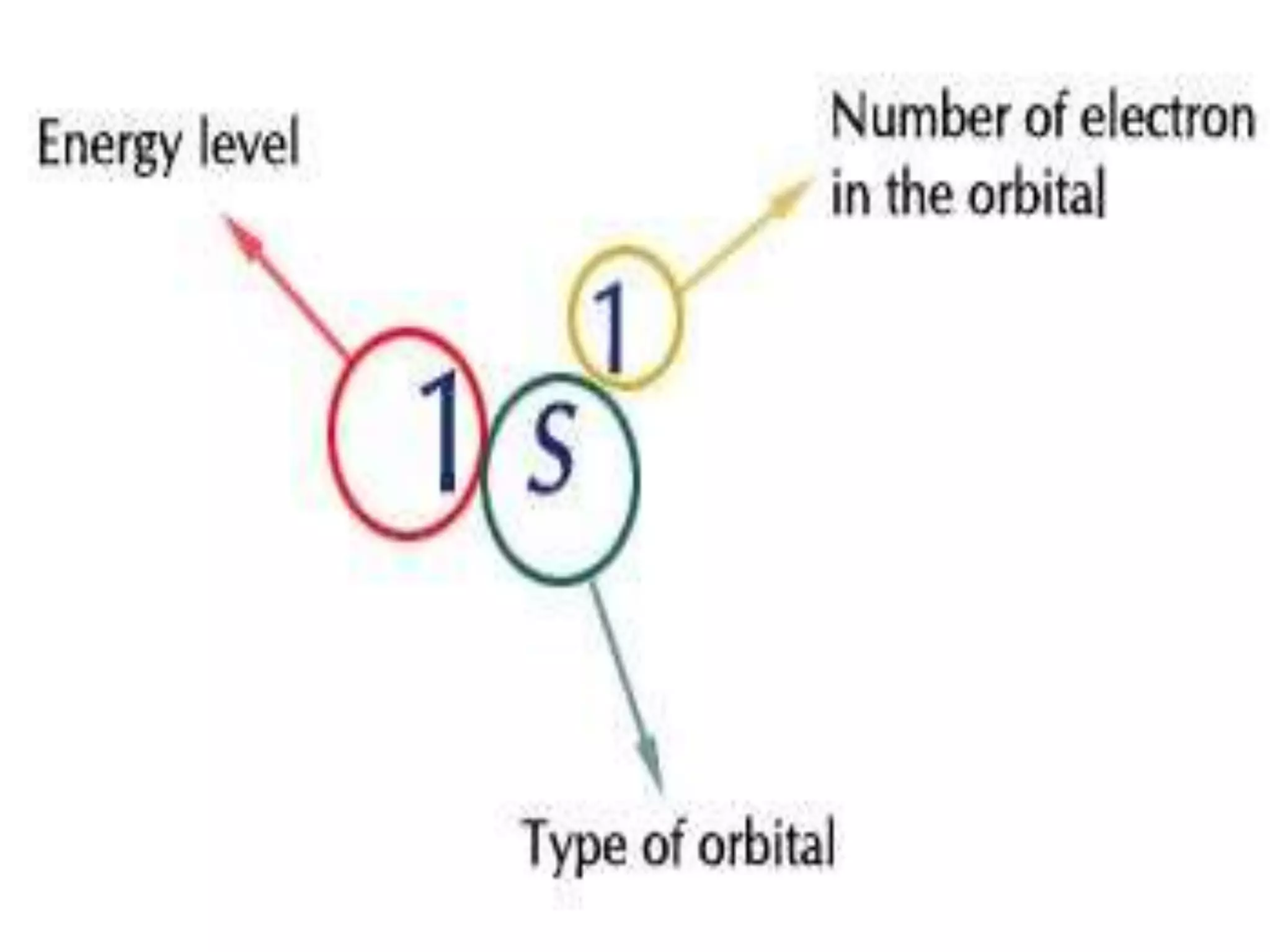
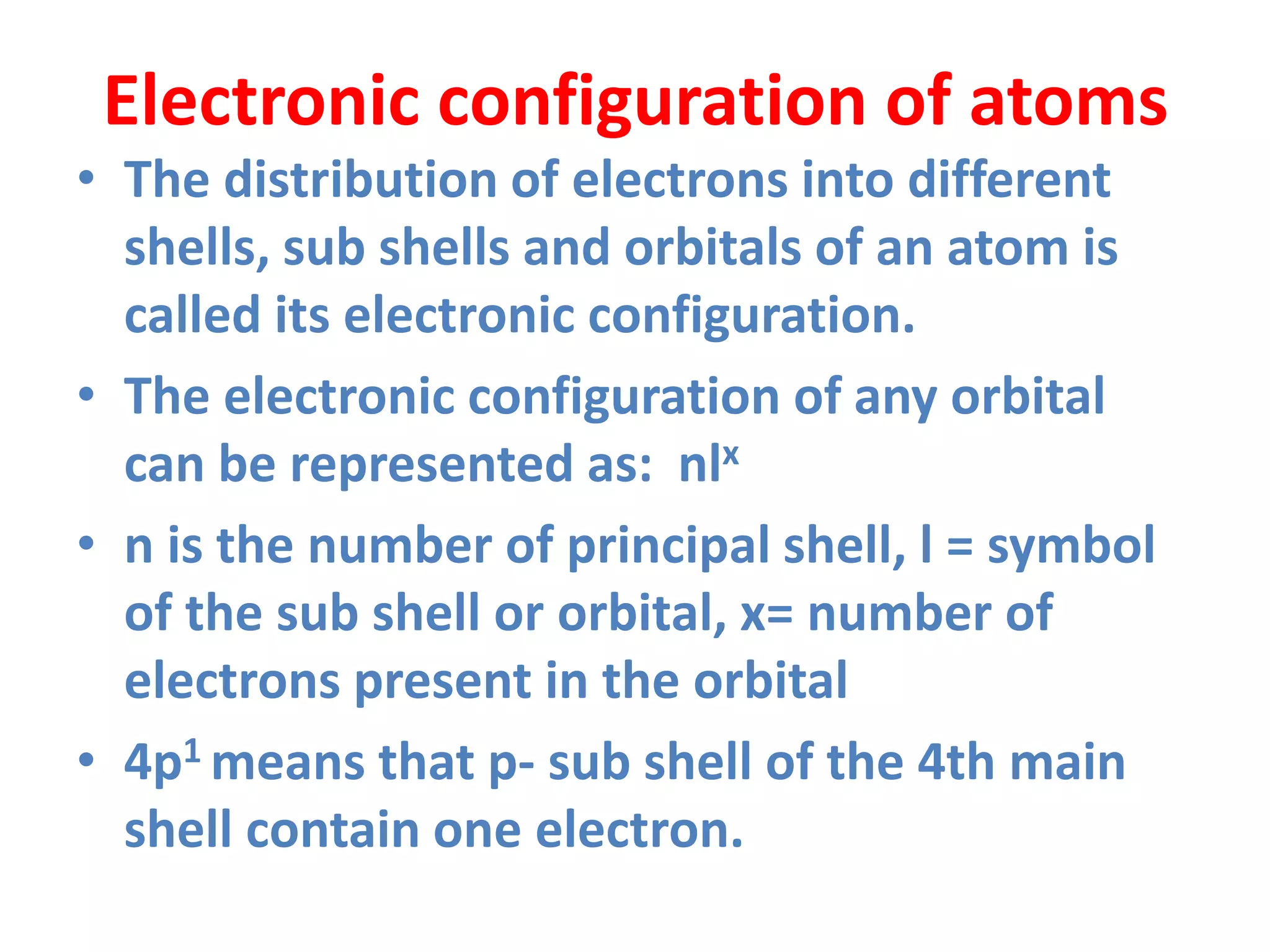
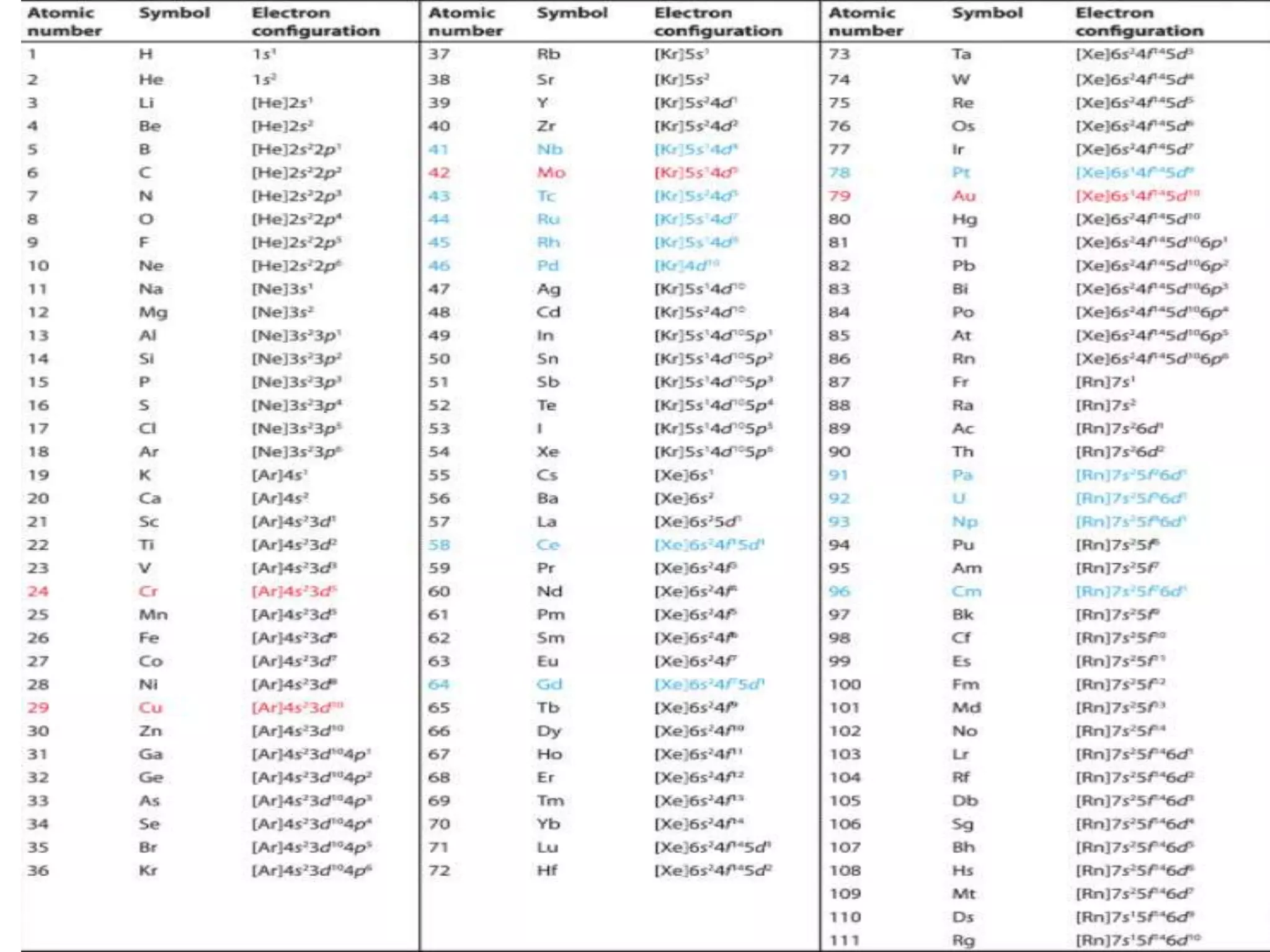
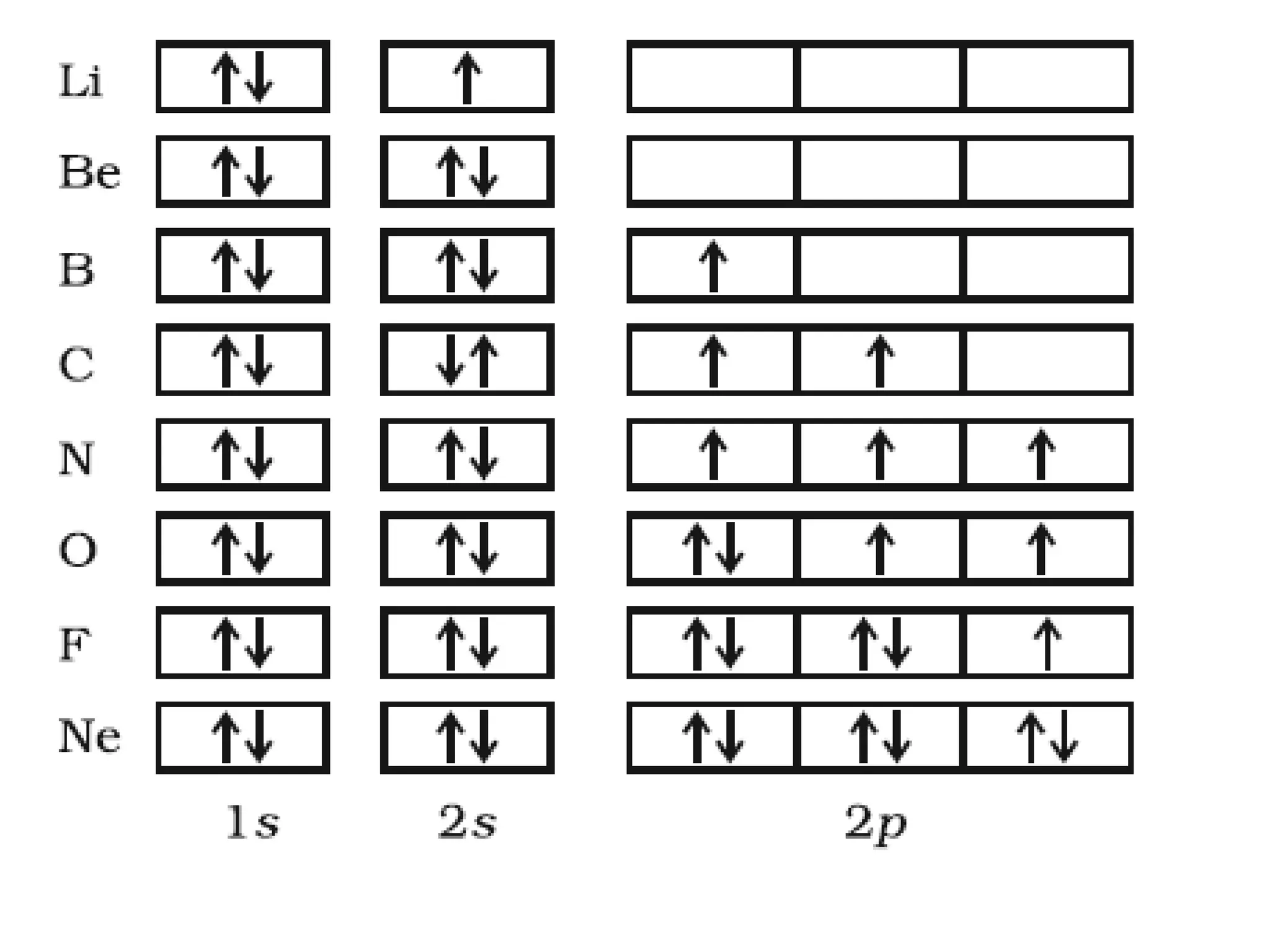
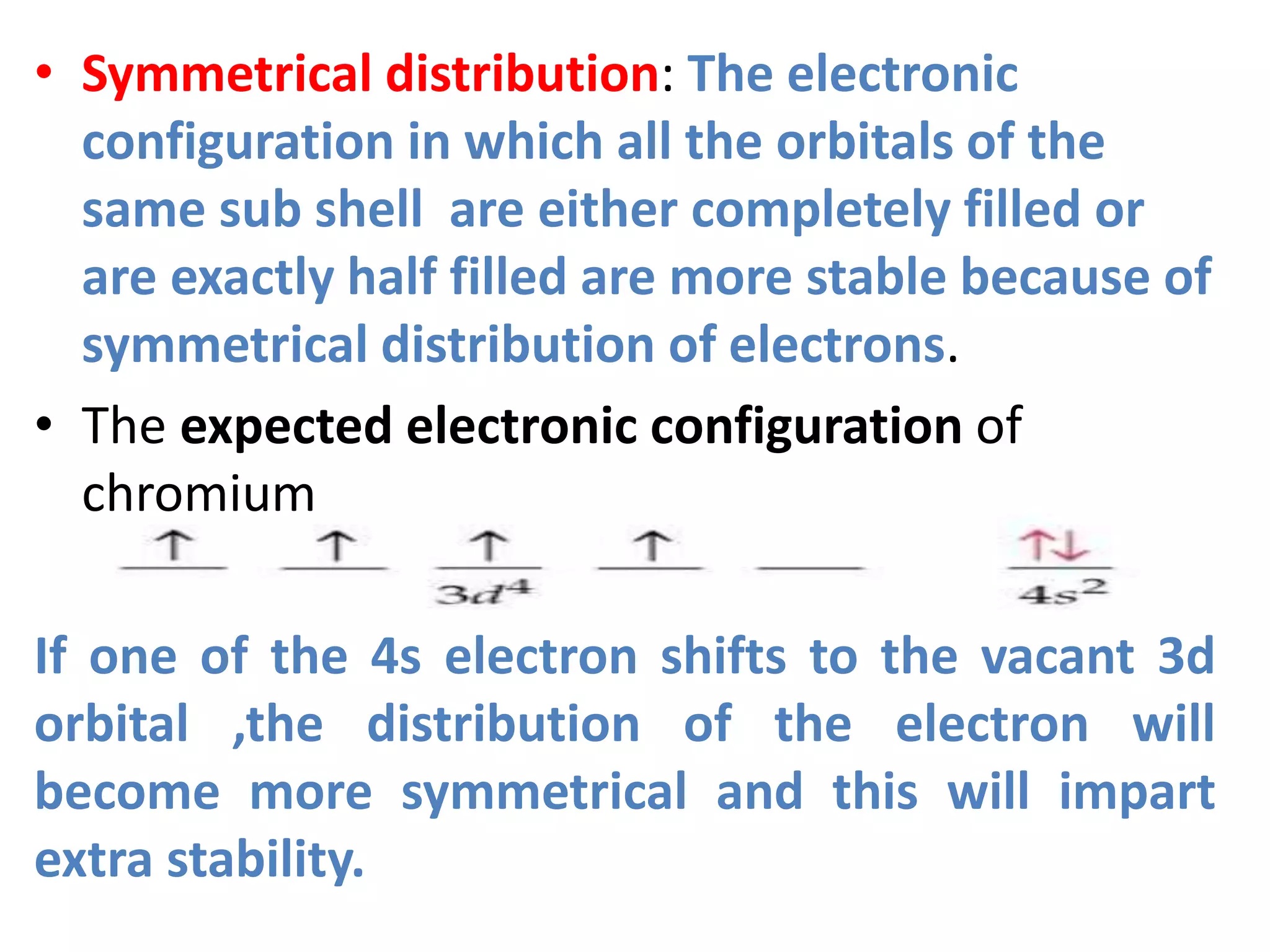
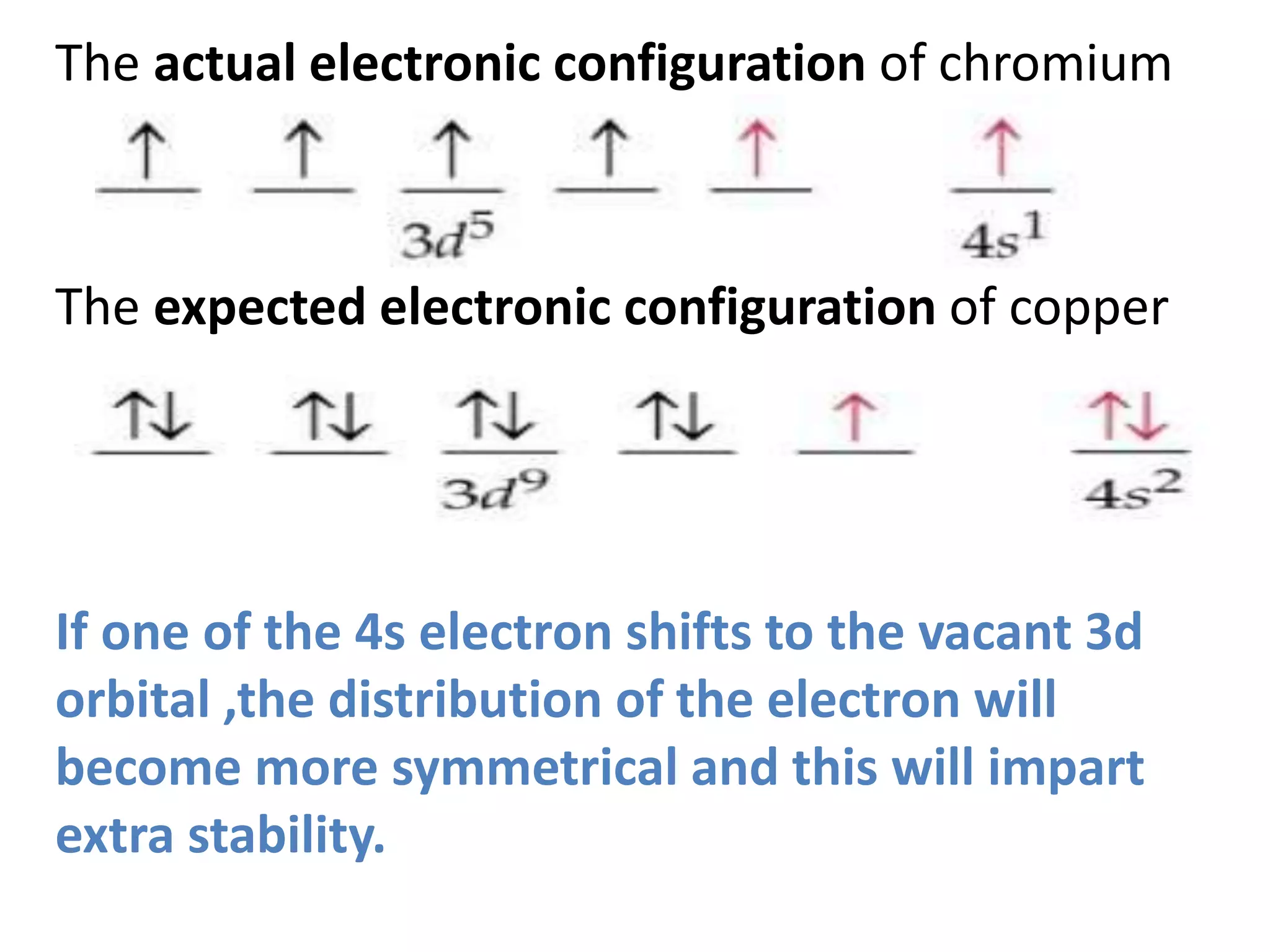
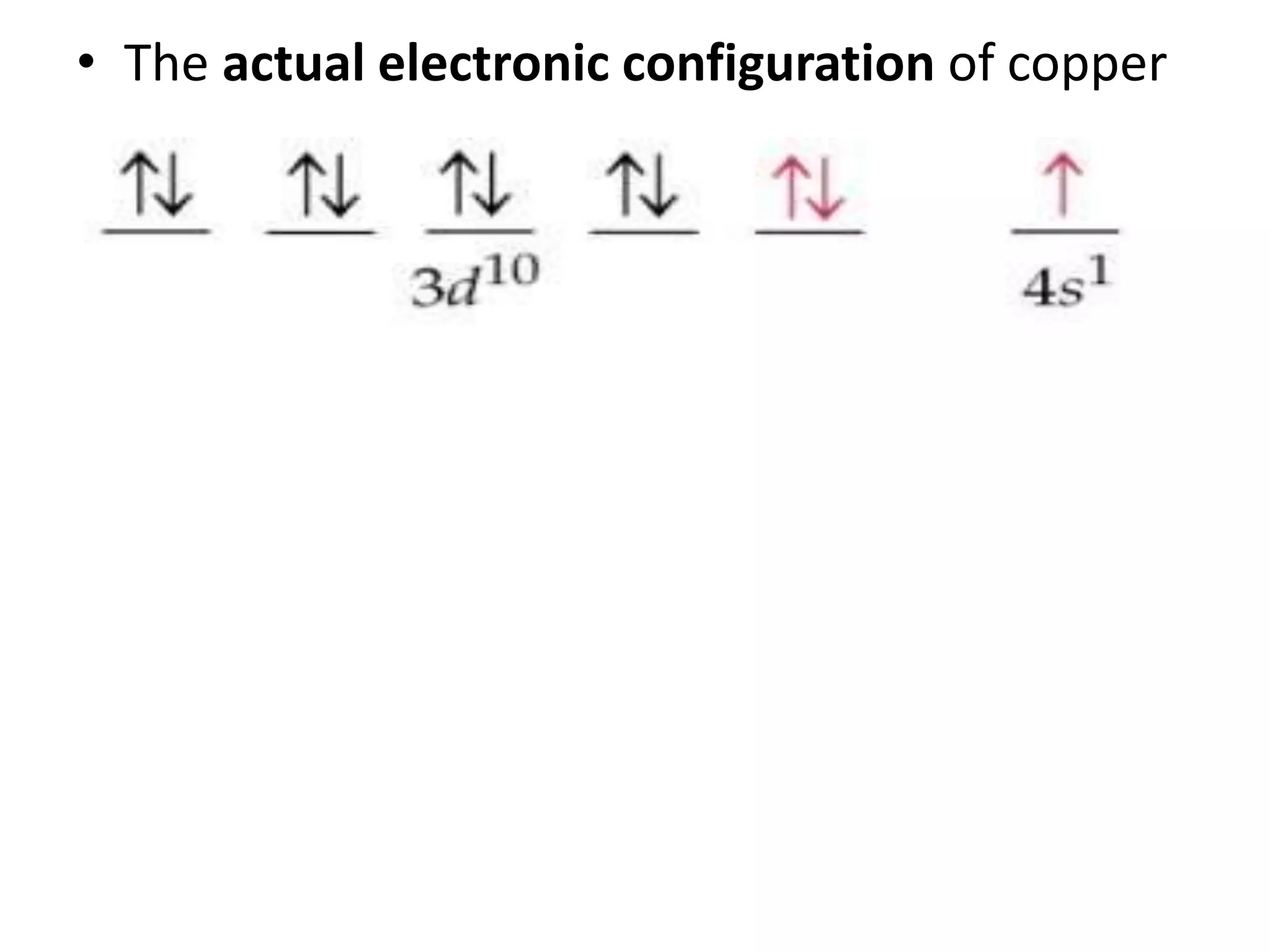
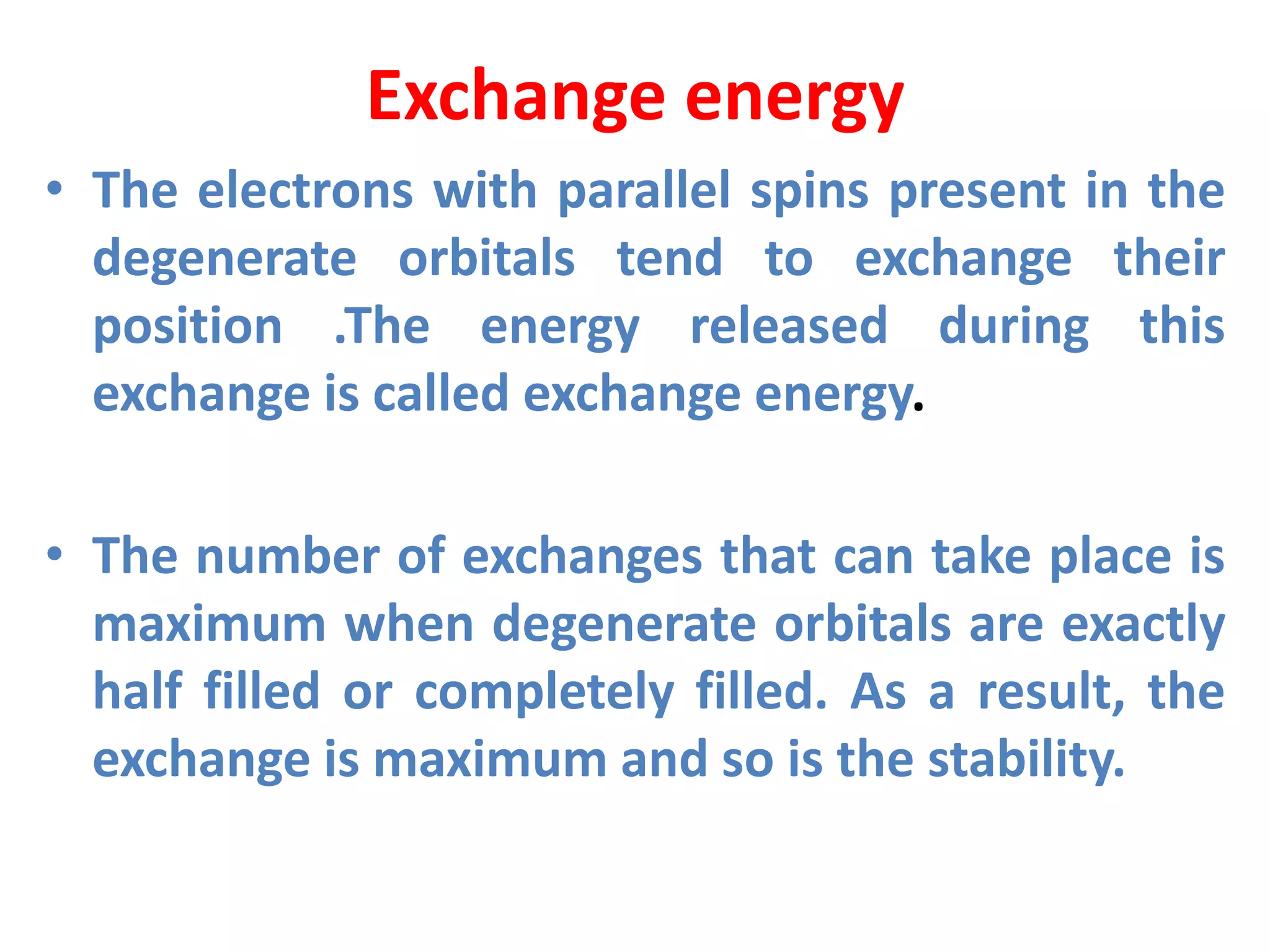
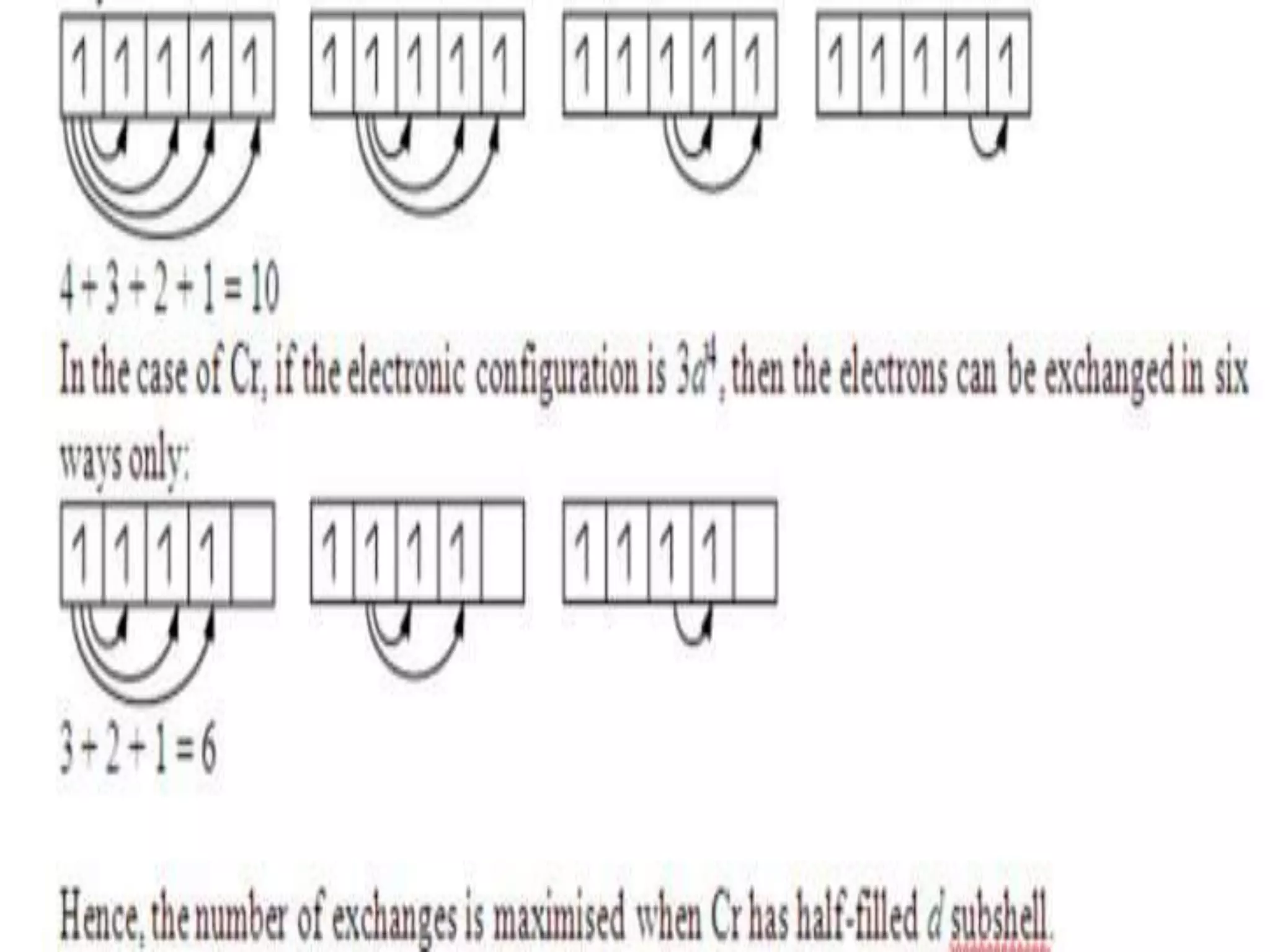
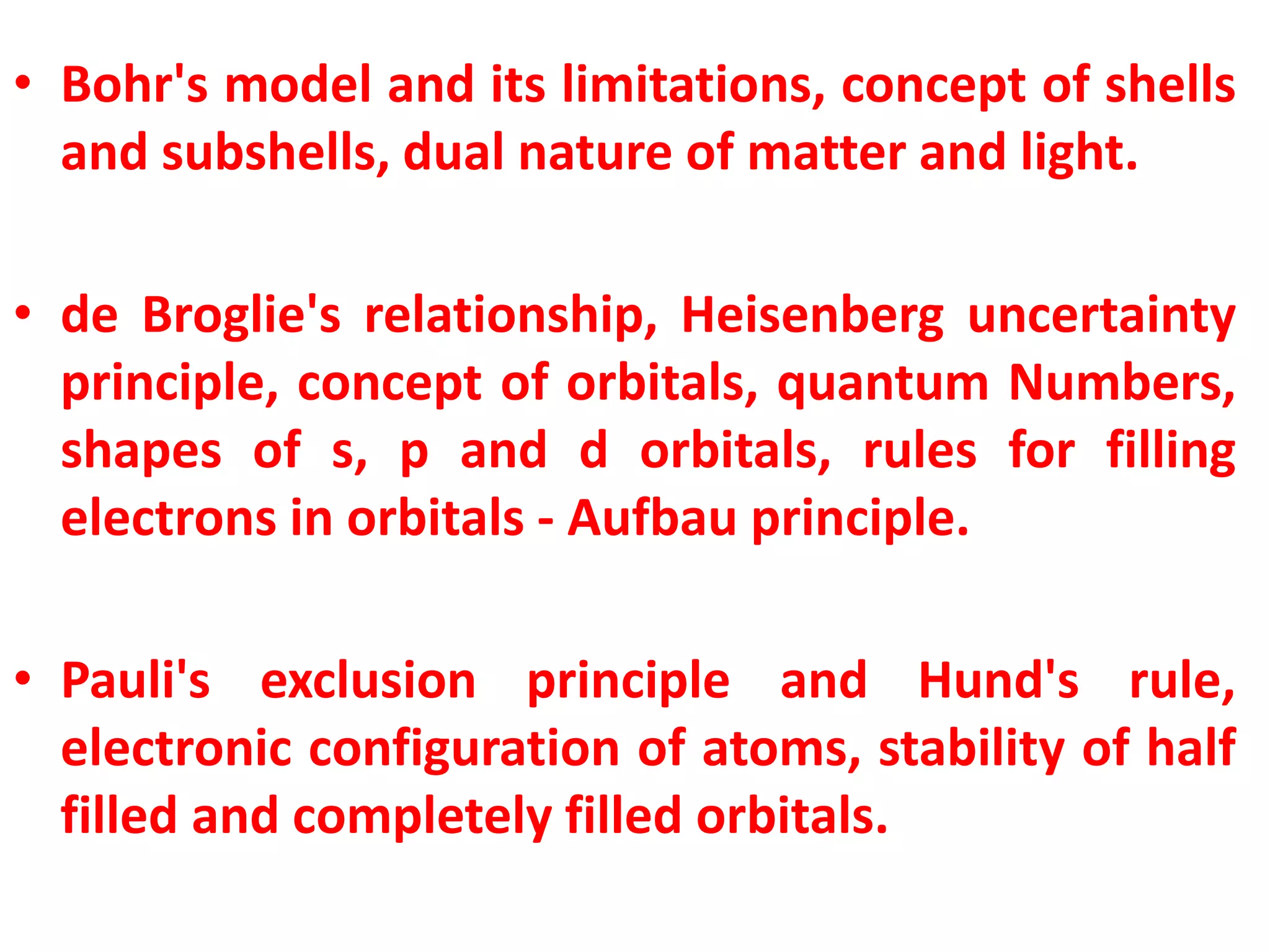
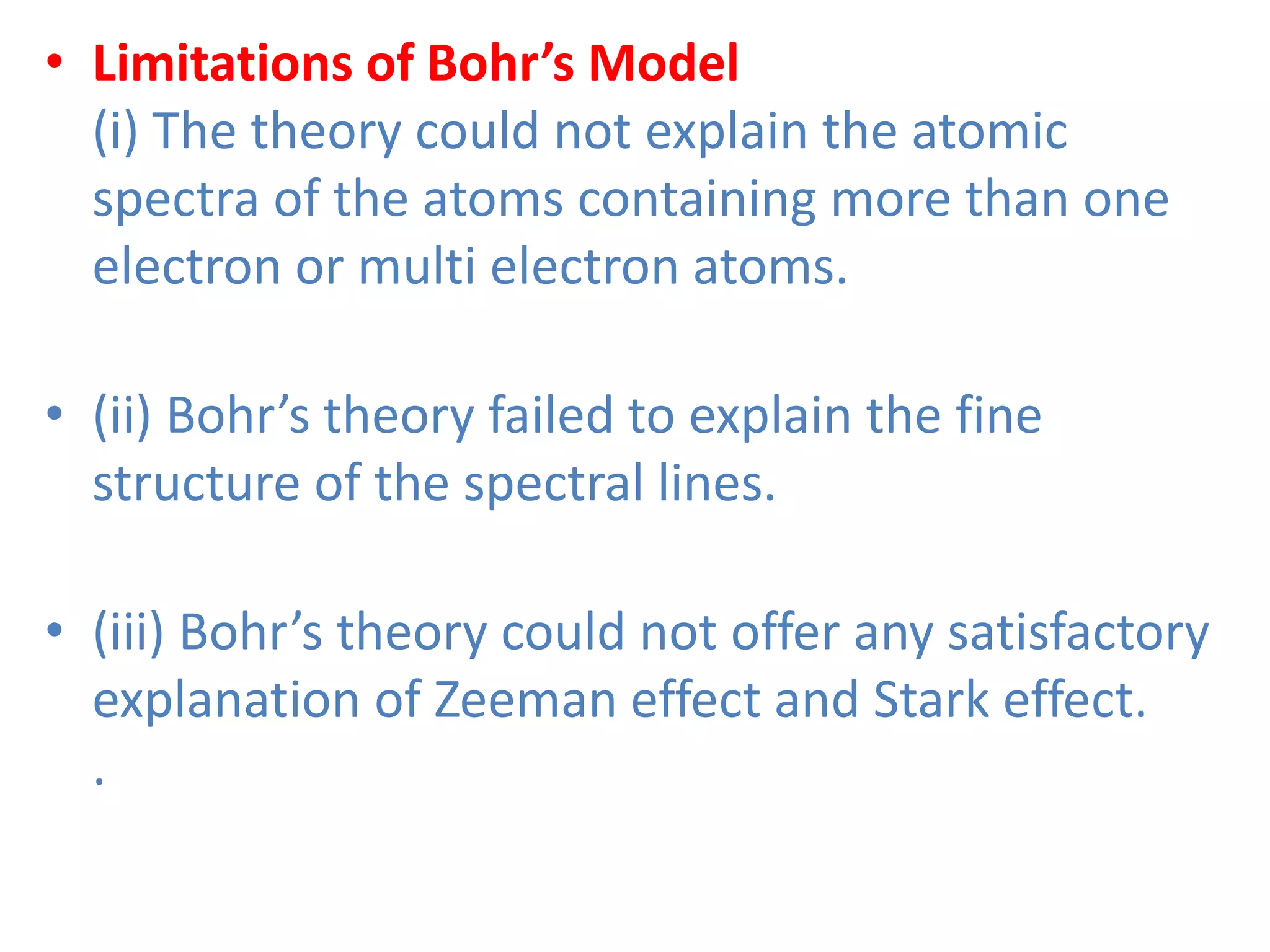
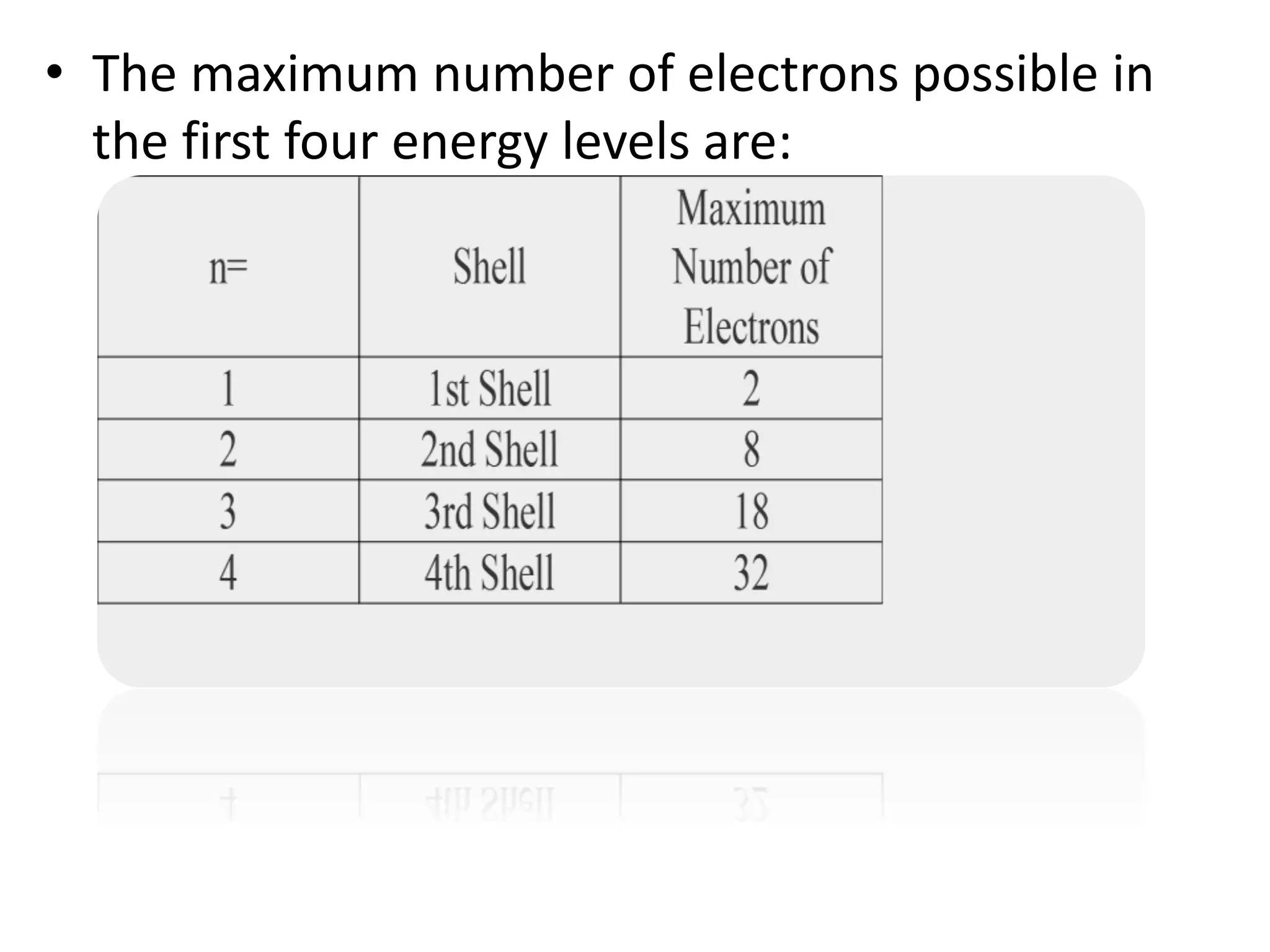
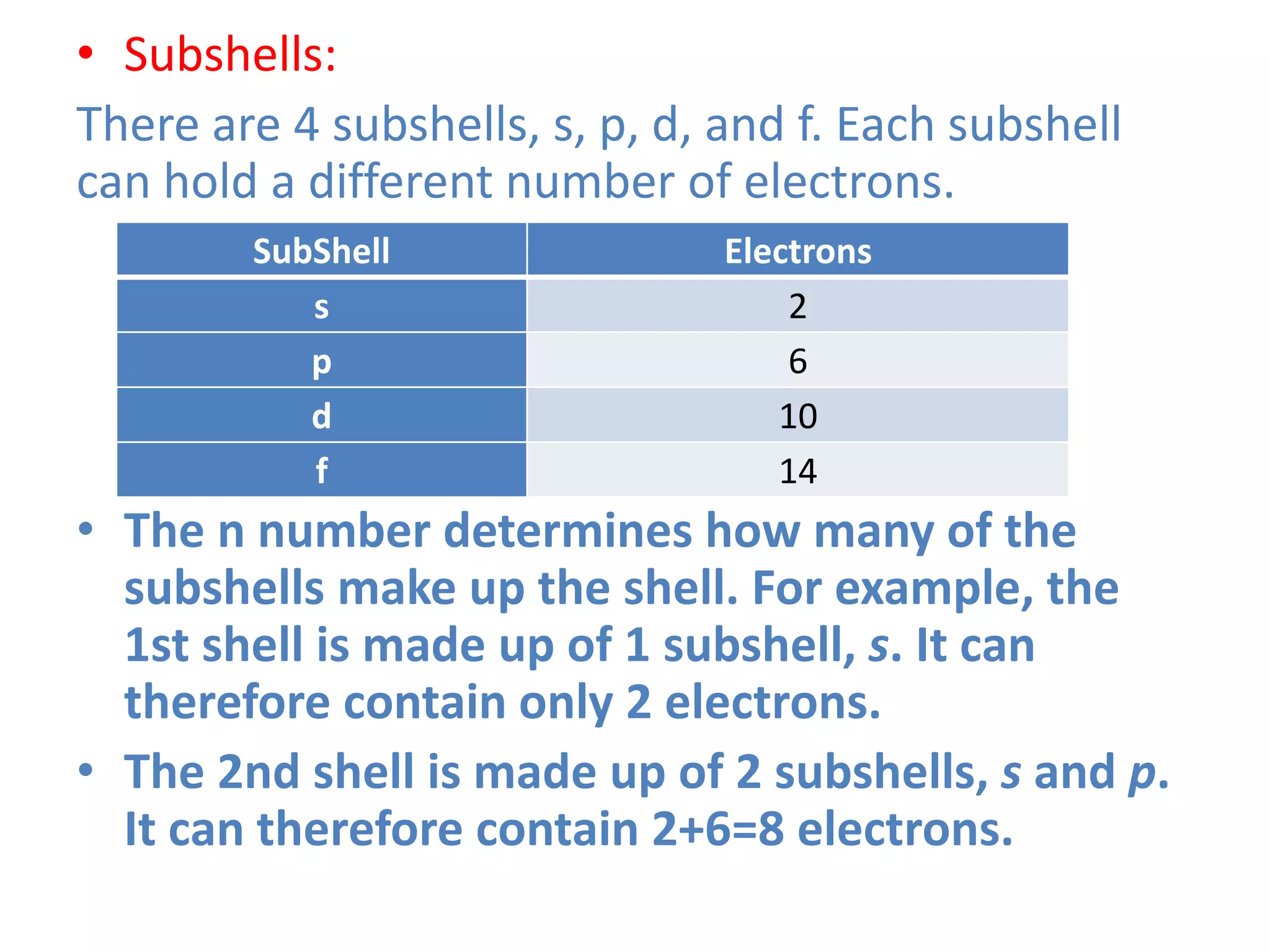
This document discusses the structure of the atom. It begins by describing Bohr's model of the atom and its limitations. It then introduces shells and subshells, as well as quantum numbers and the shapes of atomic orbitals. Rules for filling electrons into orbitals, such as the Aufbau principle and Pauli exclusion principle, are also covered. The document discusses atomic spectra, photoelectric effect, and the dual wave-particle nature of light and matter. It provides an overview of concepts like de Broglie wavelength, Heisenberg uncertainty principle, and atomic electron configuration.










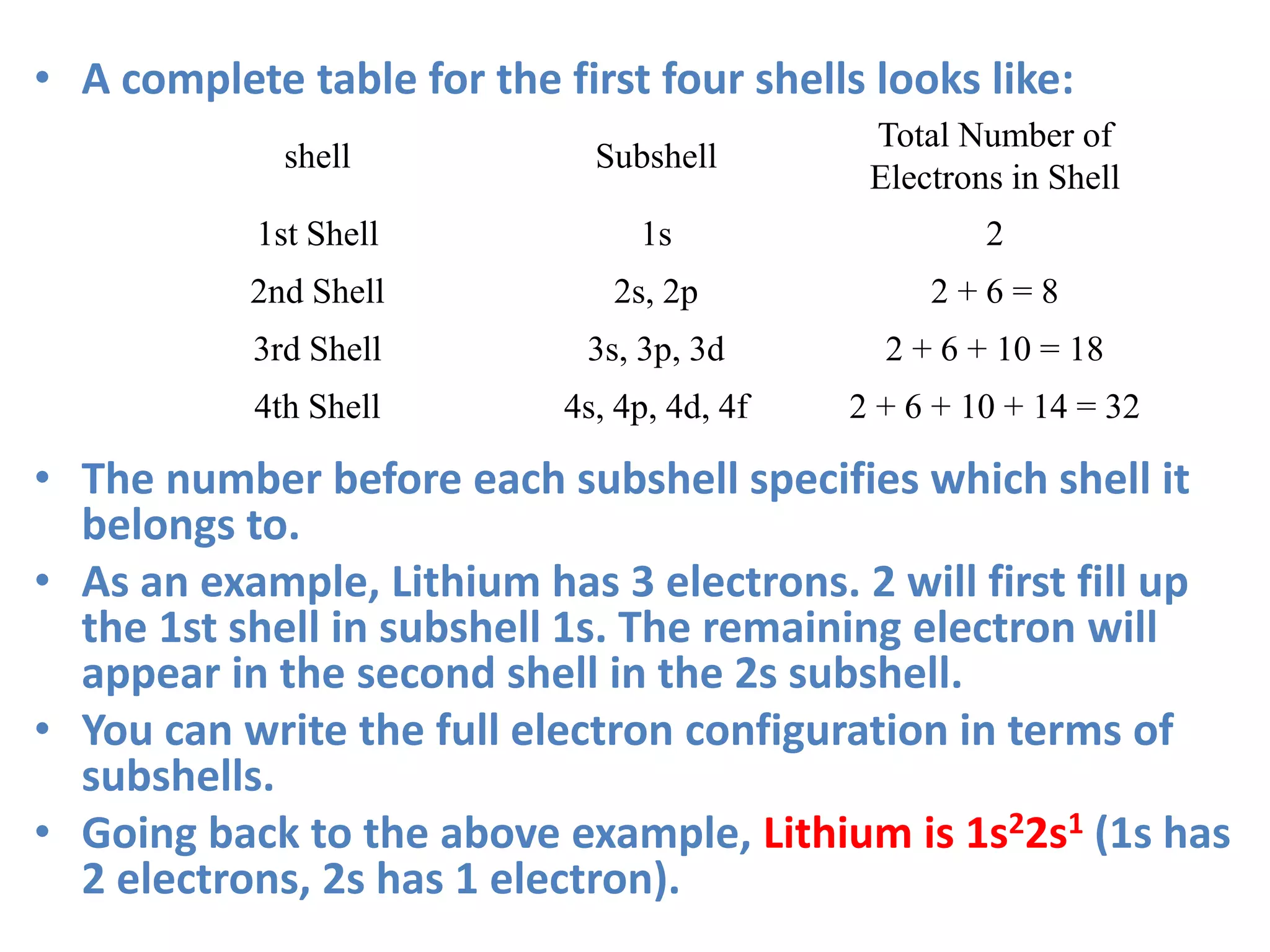
![• Lithium 1s22s1 can be simplified to [He]2s1 as
Helium (He) has an electron configuration of
1s2.
• Note: subshells have different energy levels
which can confuse the order they fill.](https://image.slidesharecdn.com/structureofatom-201129083435/75/Structure-of-atom-21-2048.jpg)
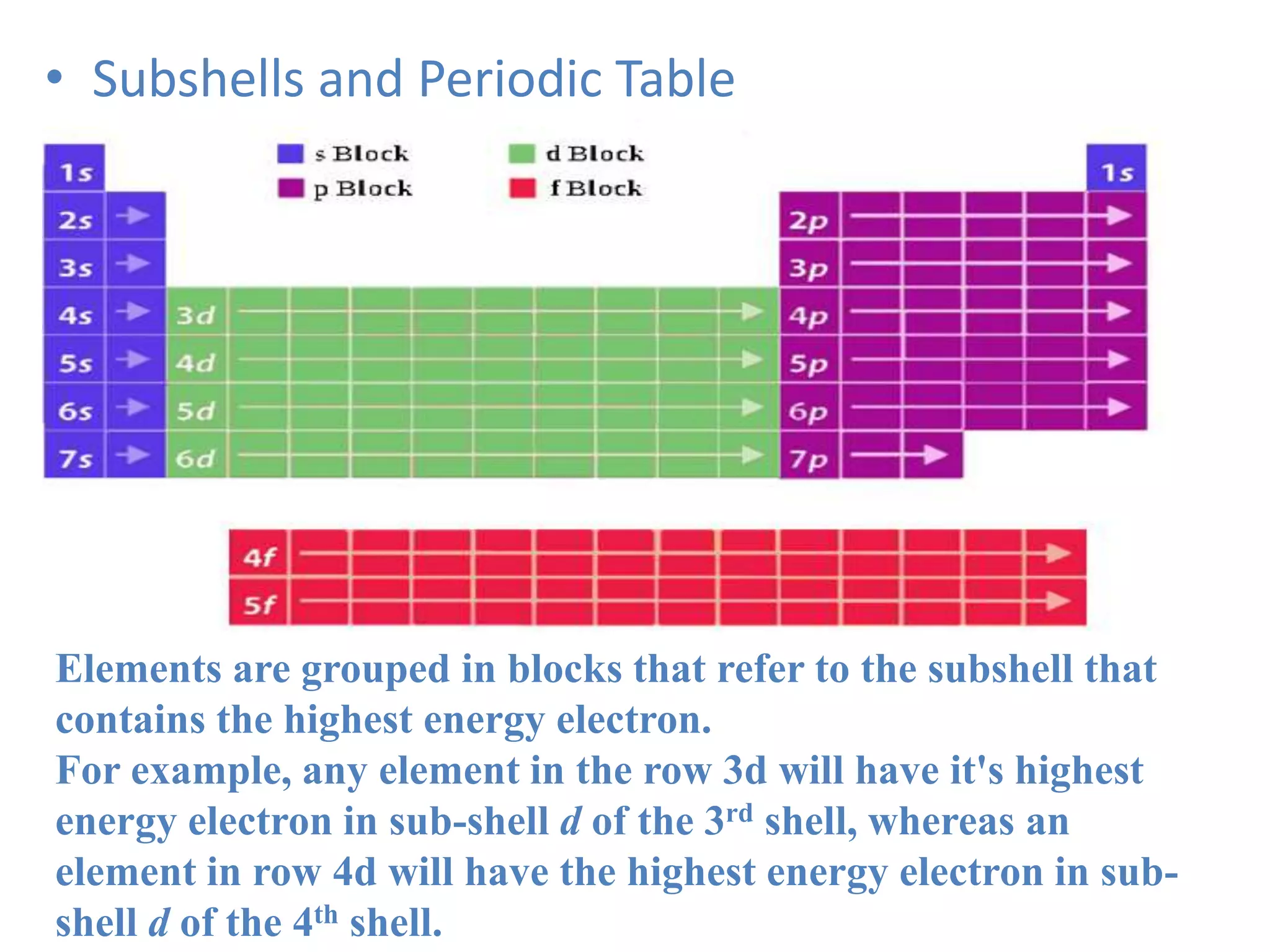


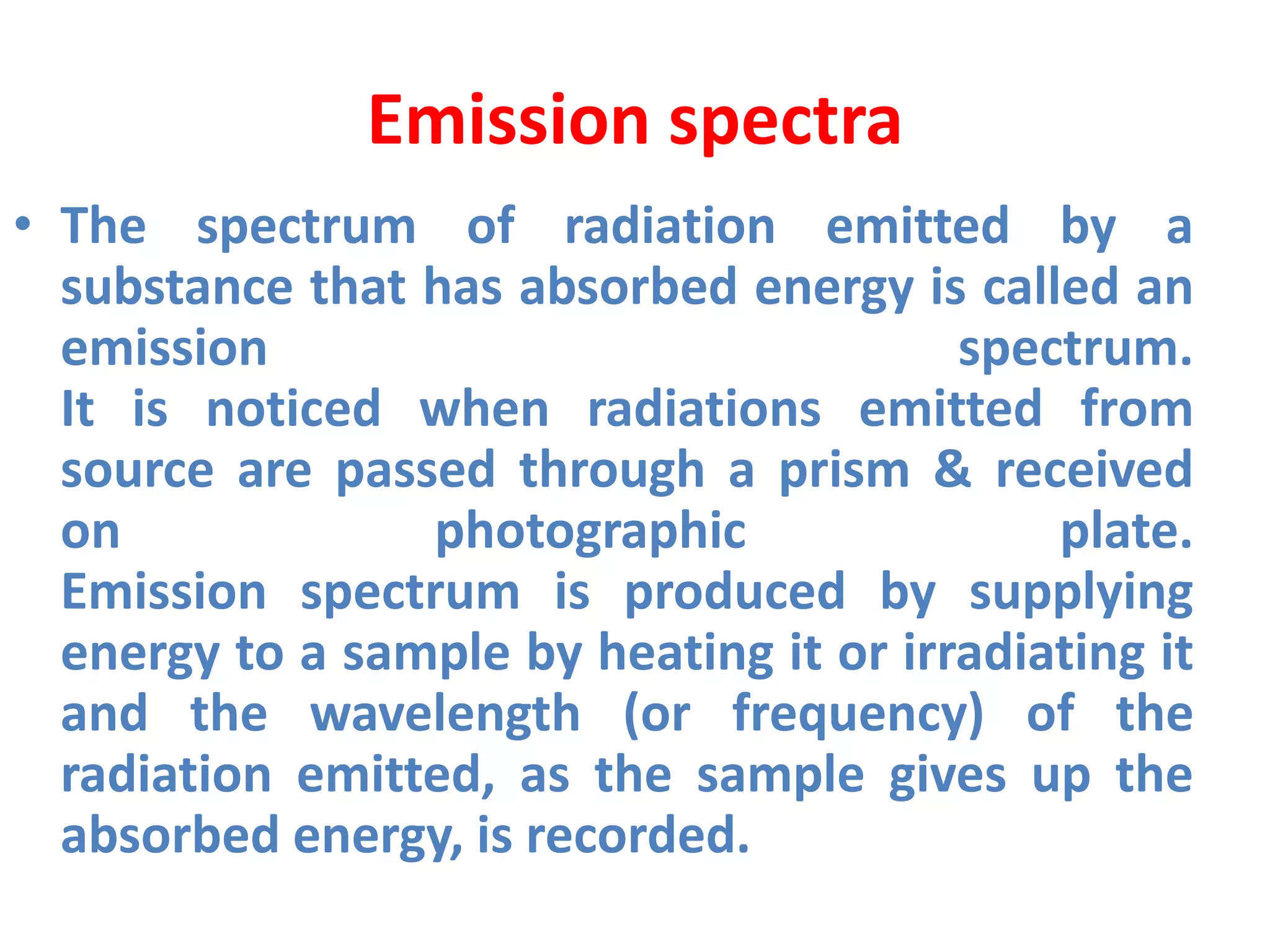
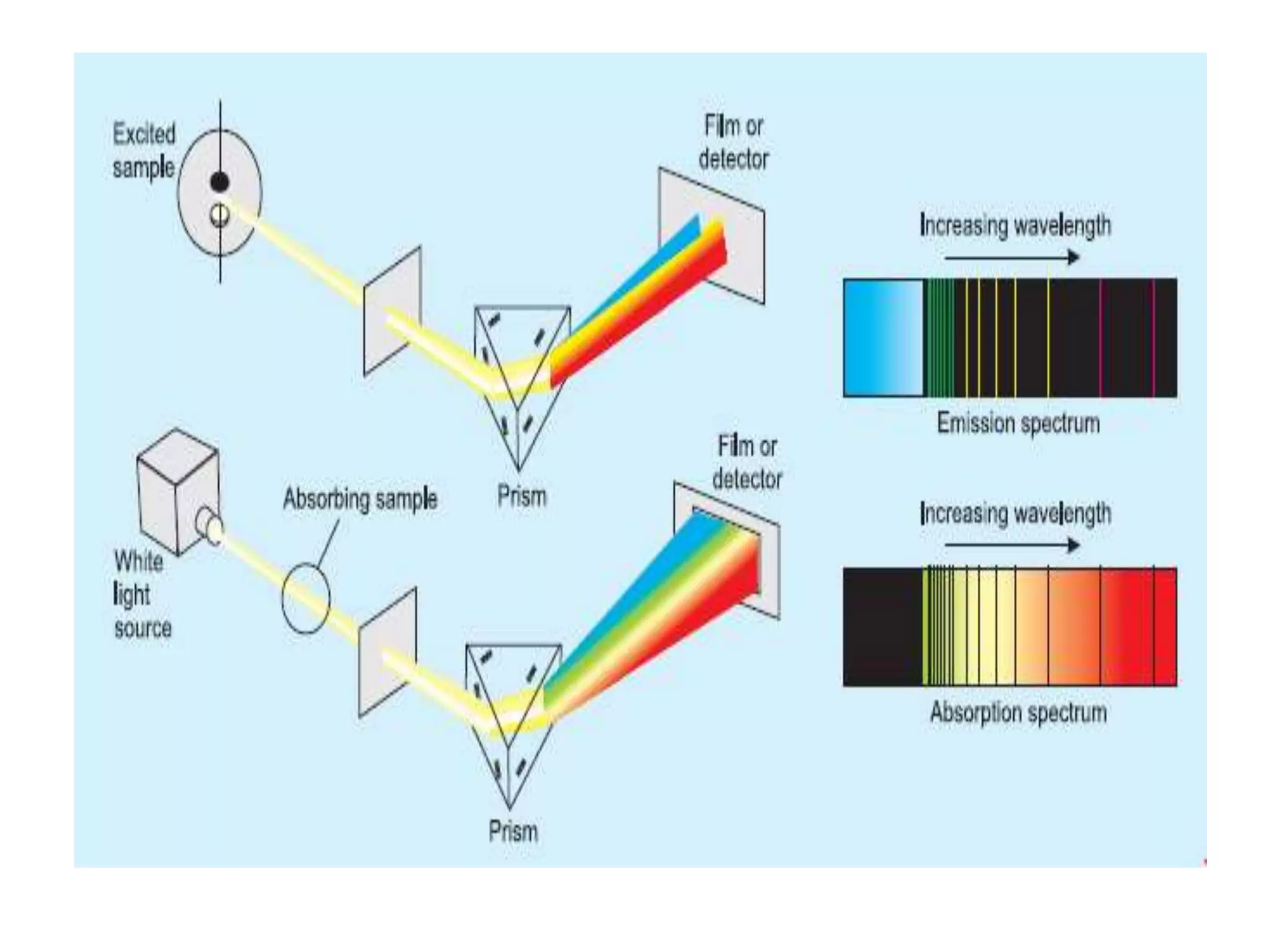

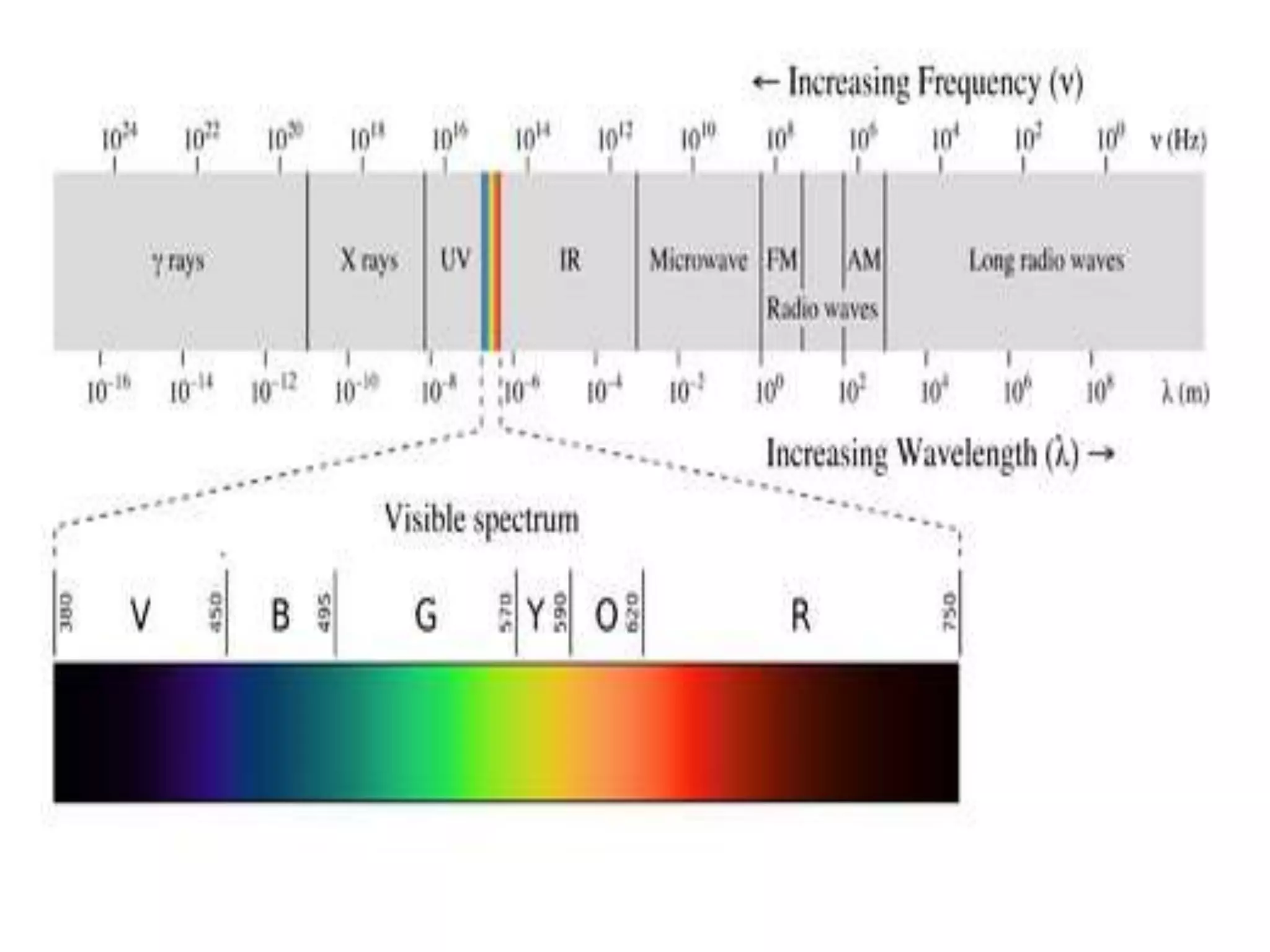
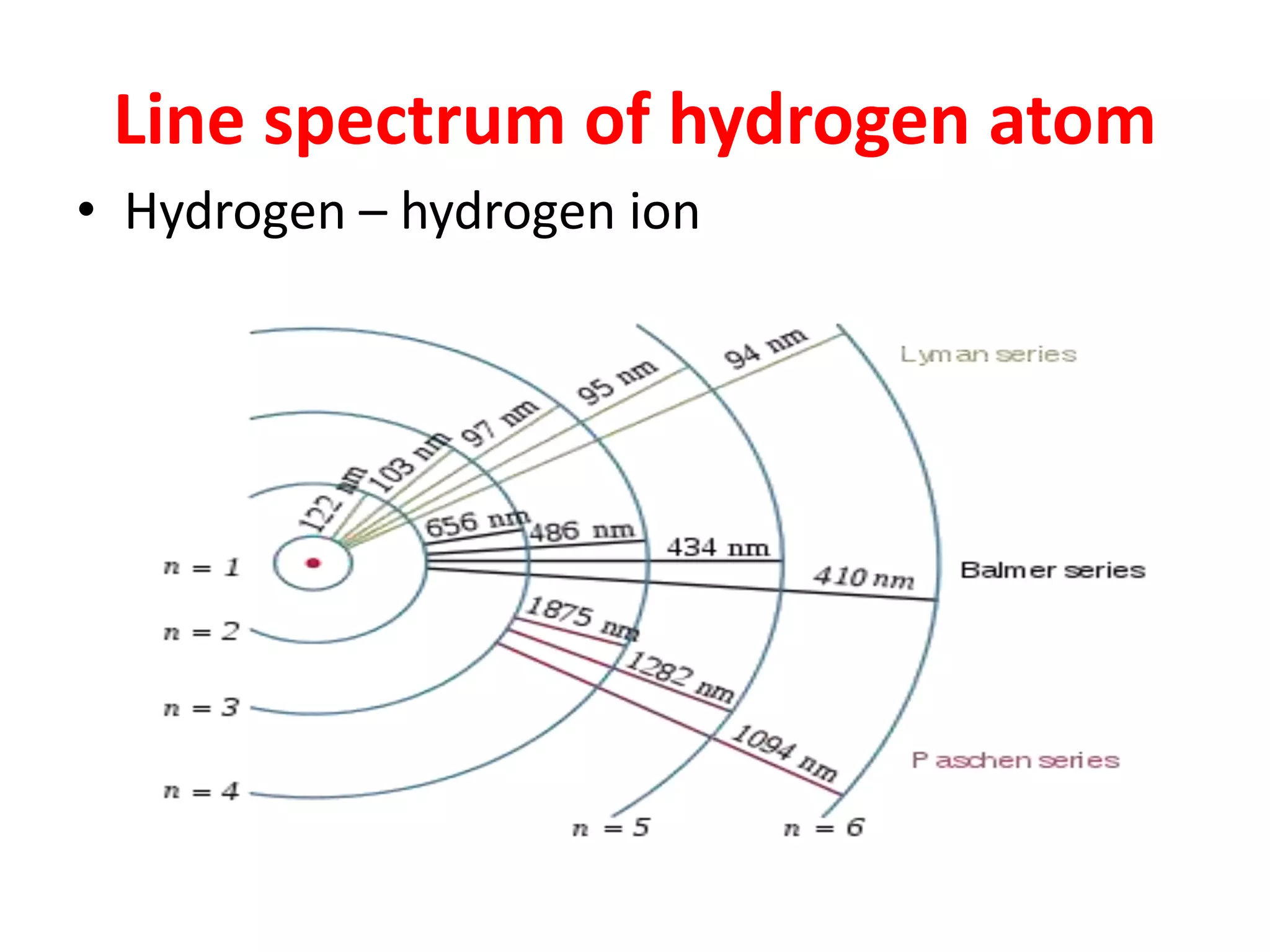
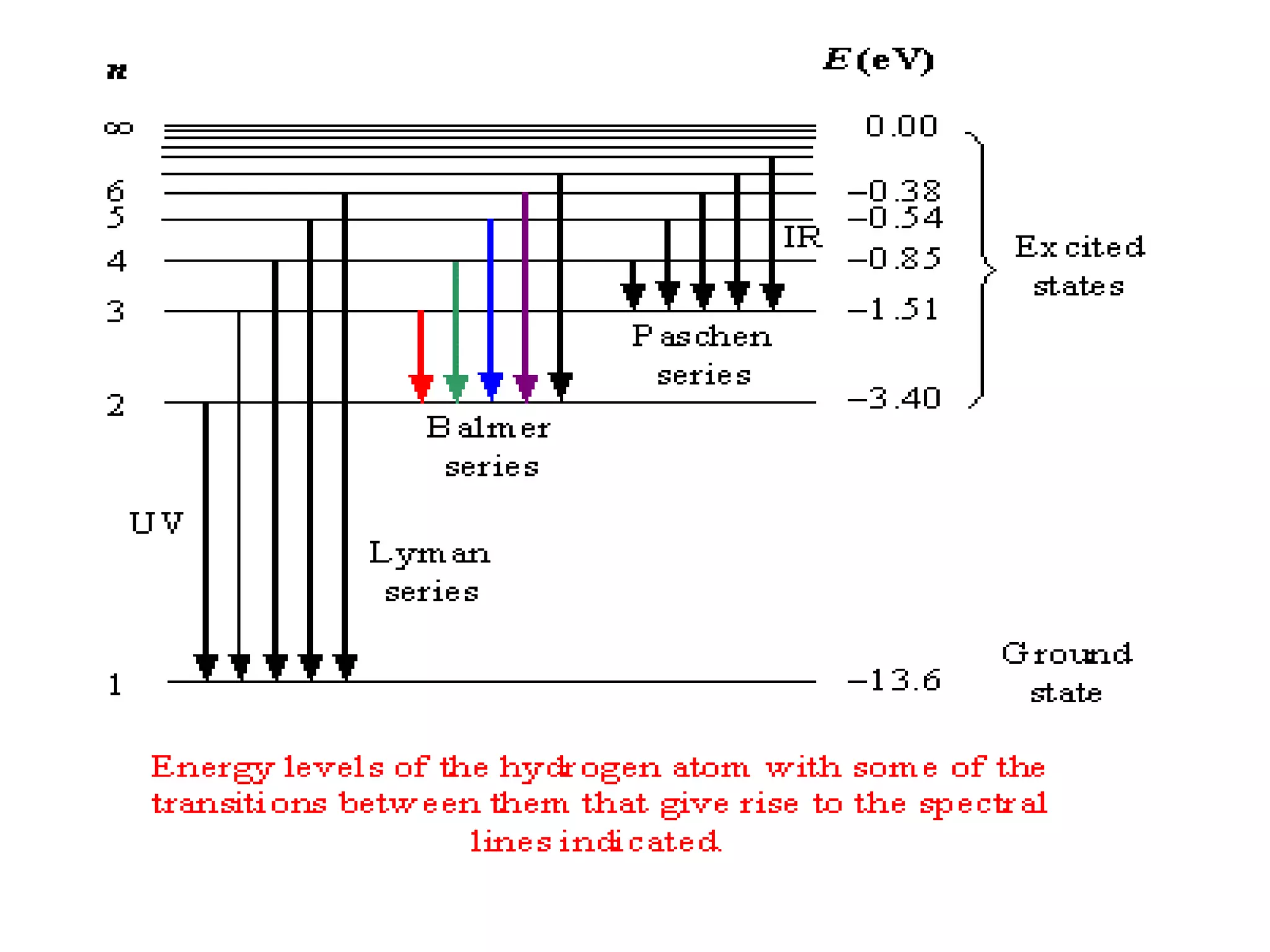

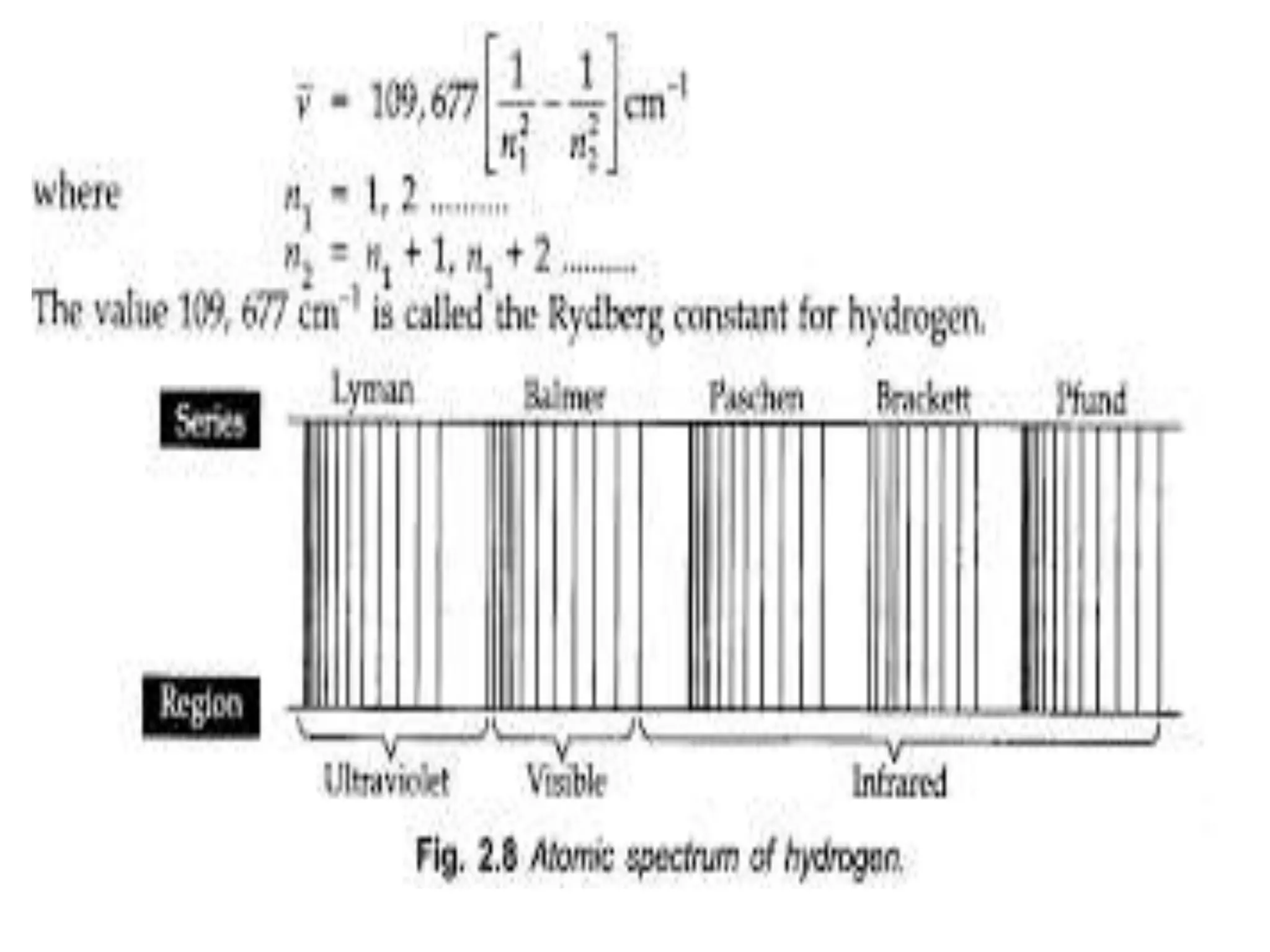





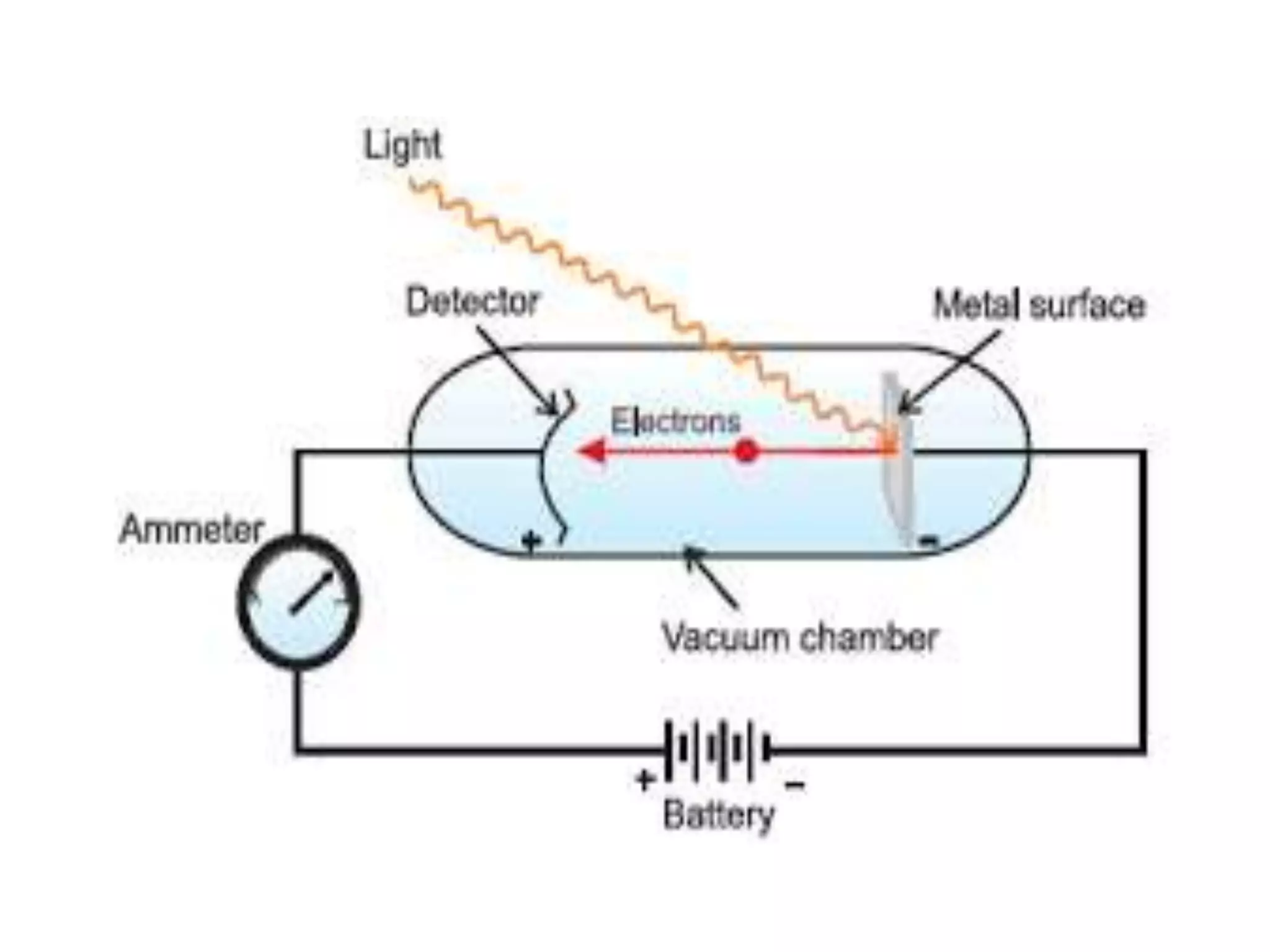
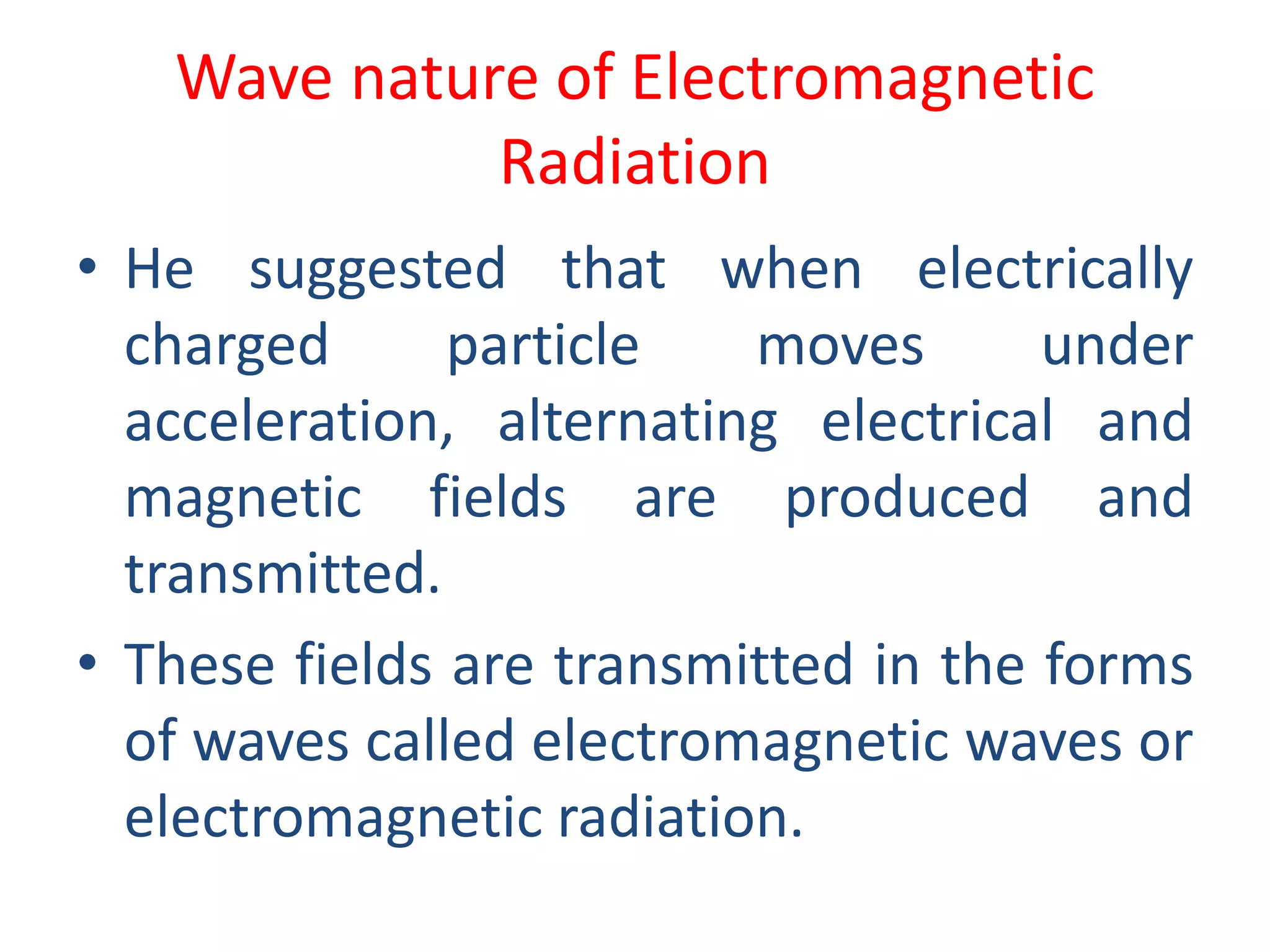

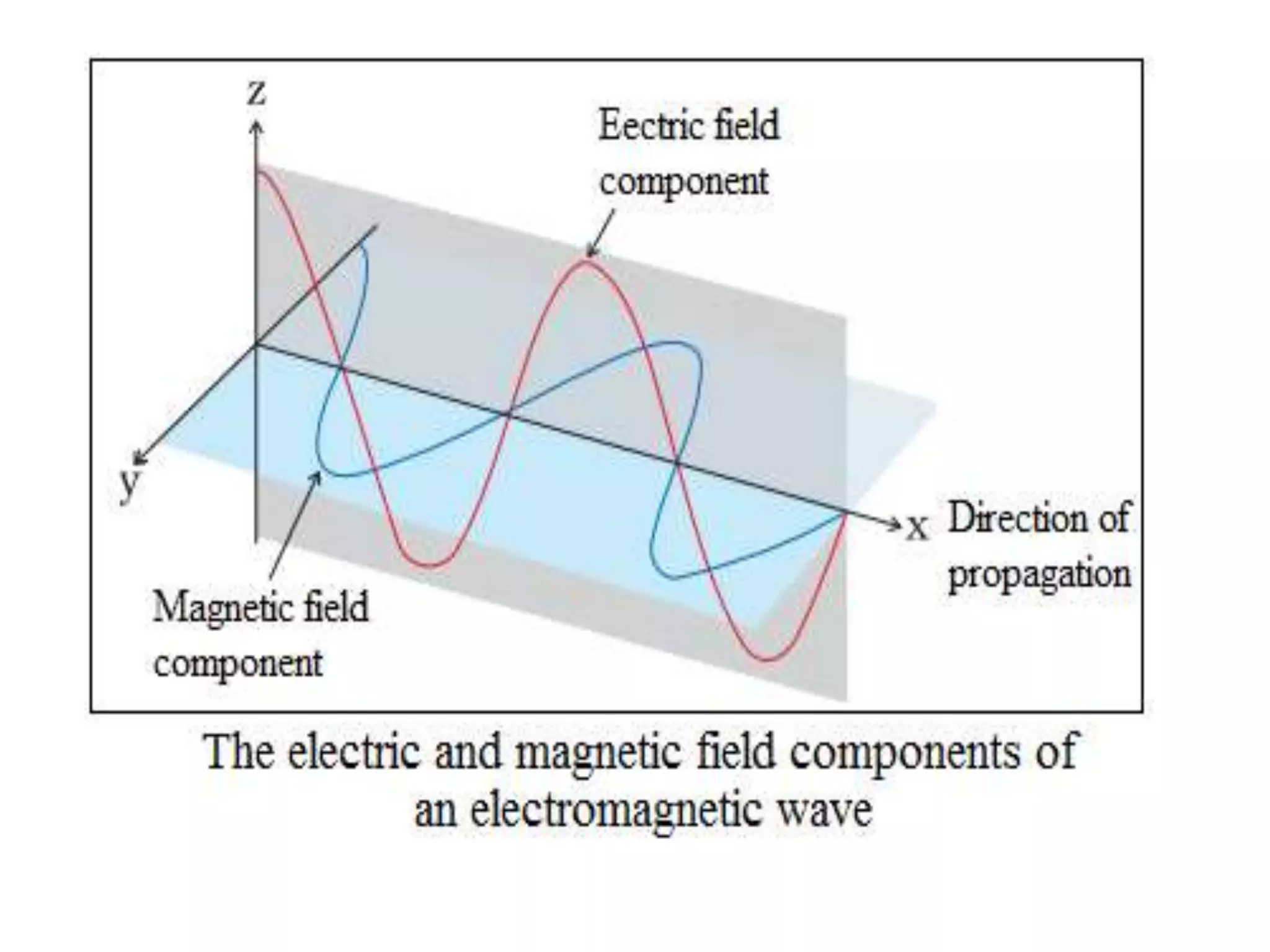





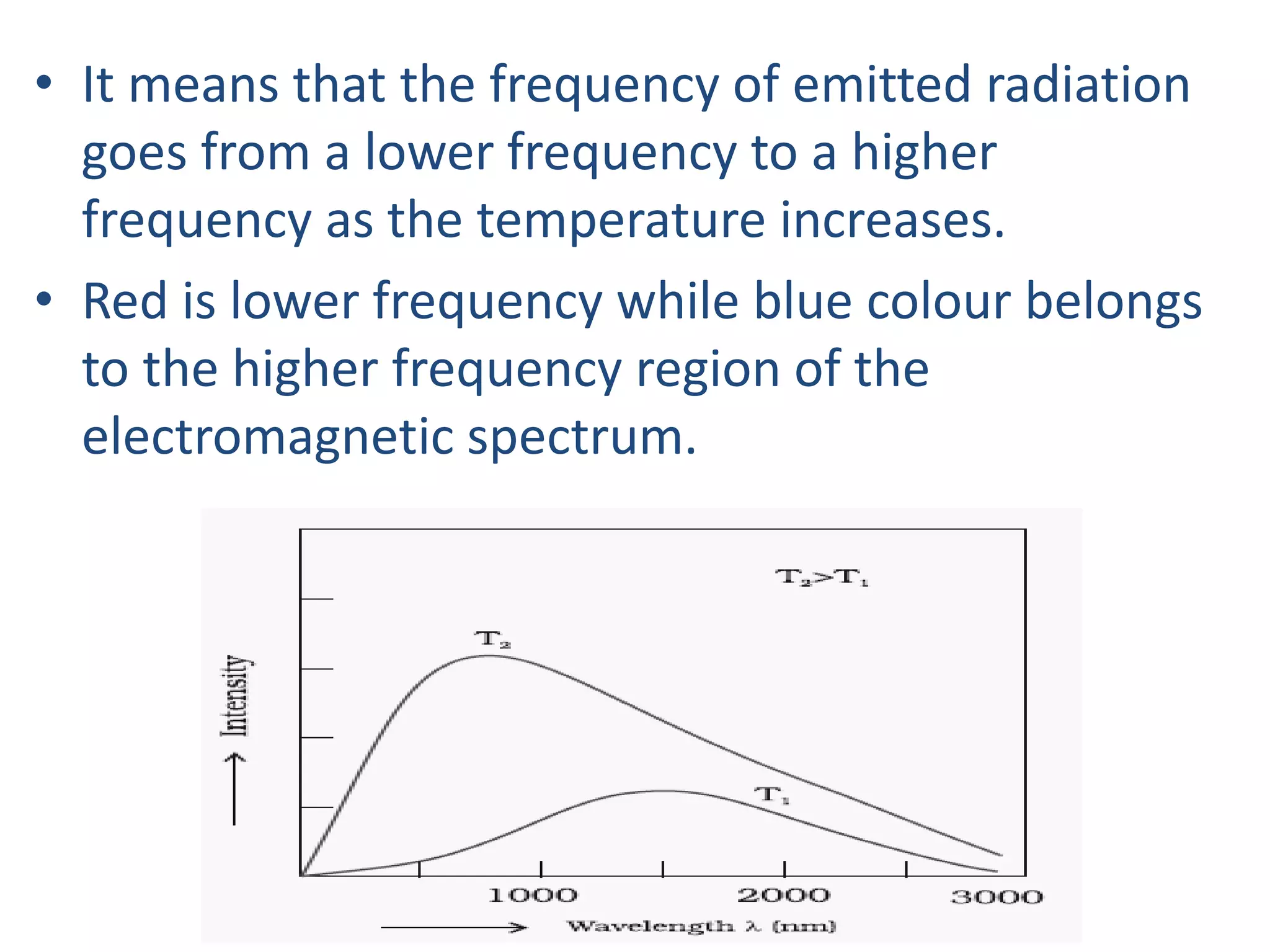




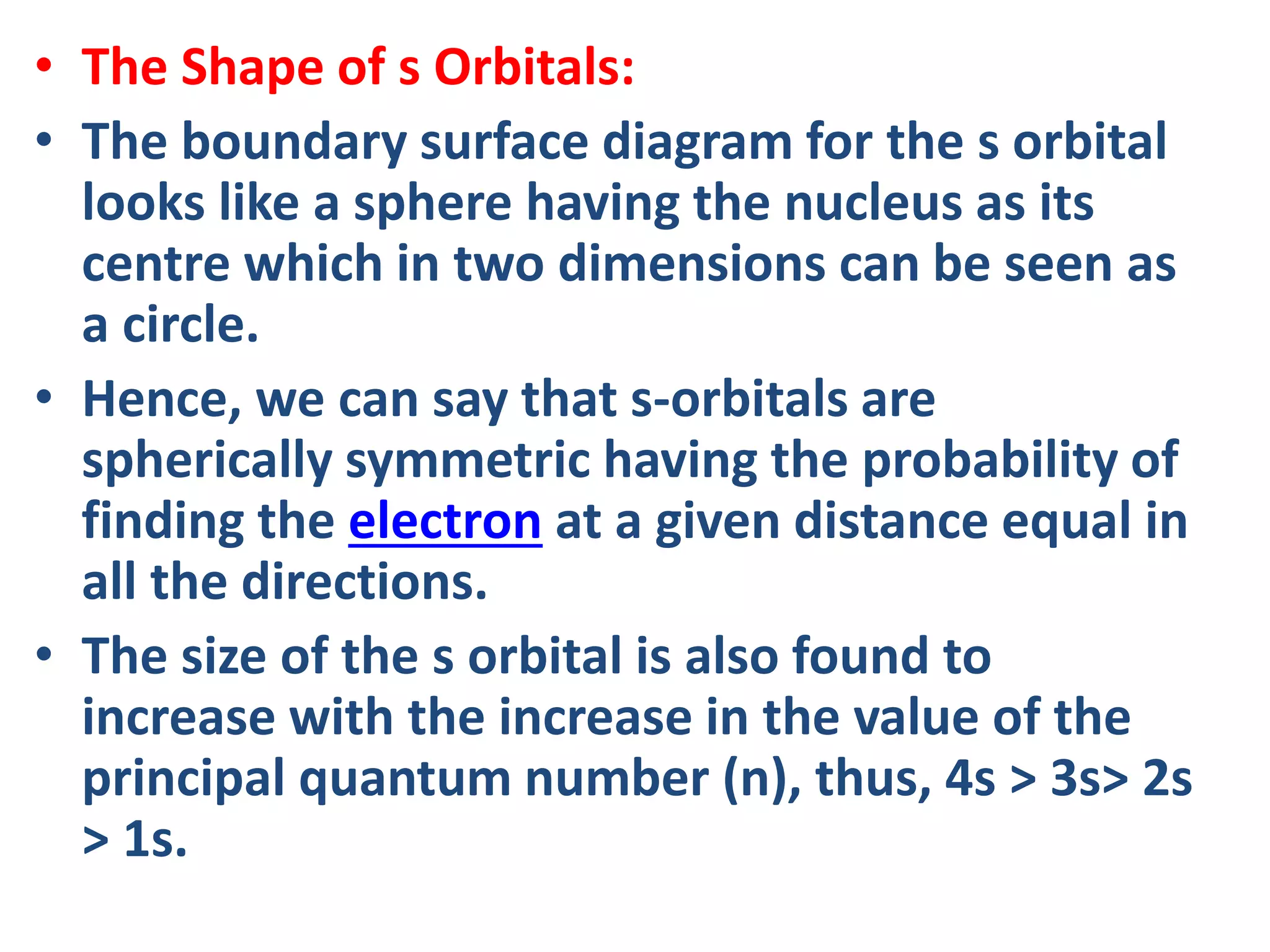
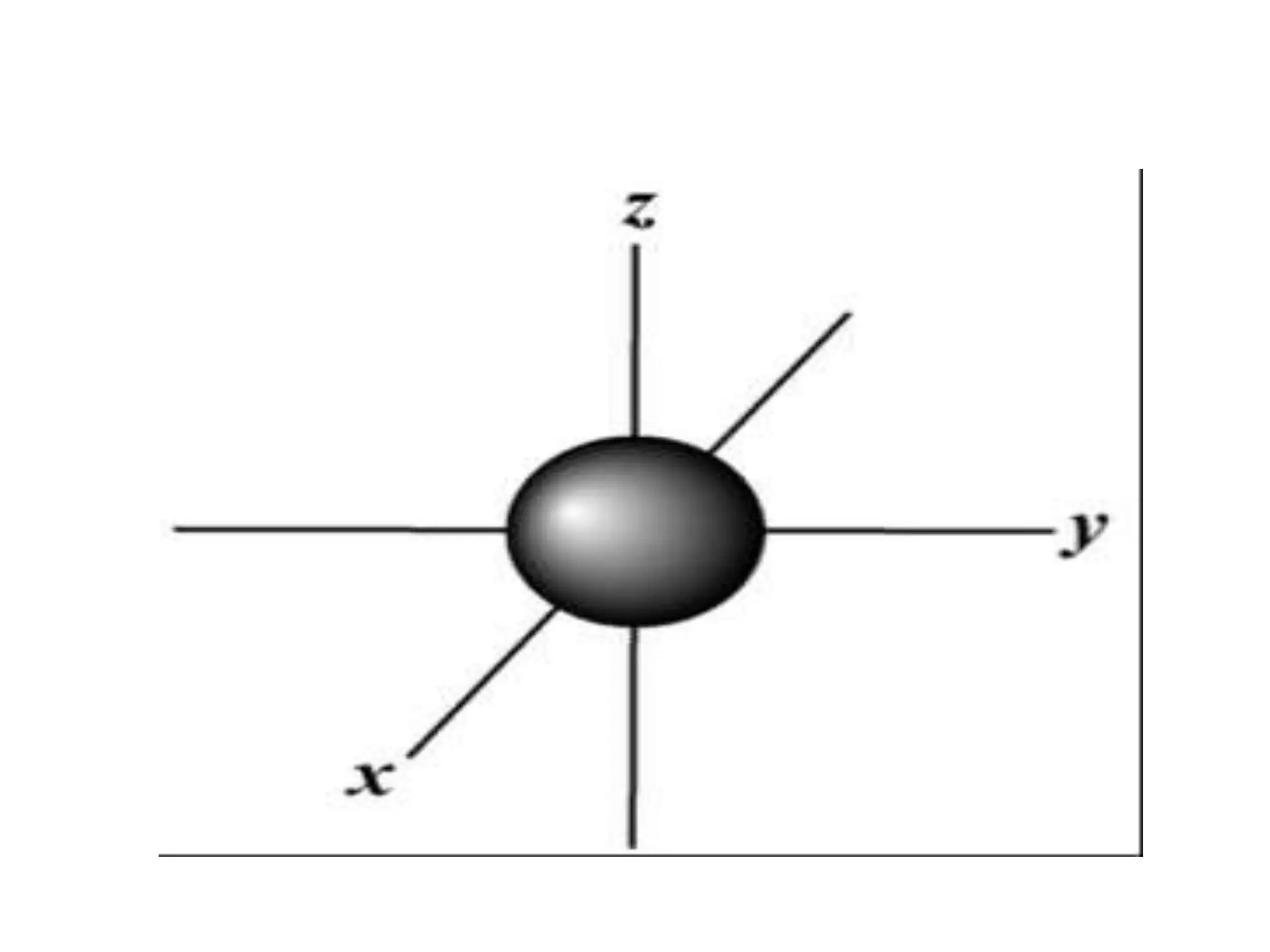
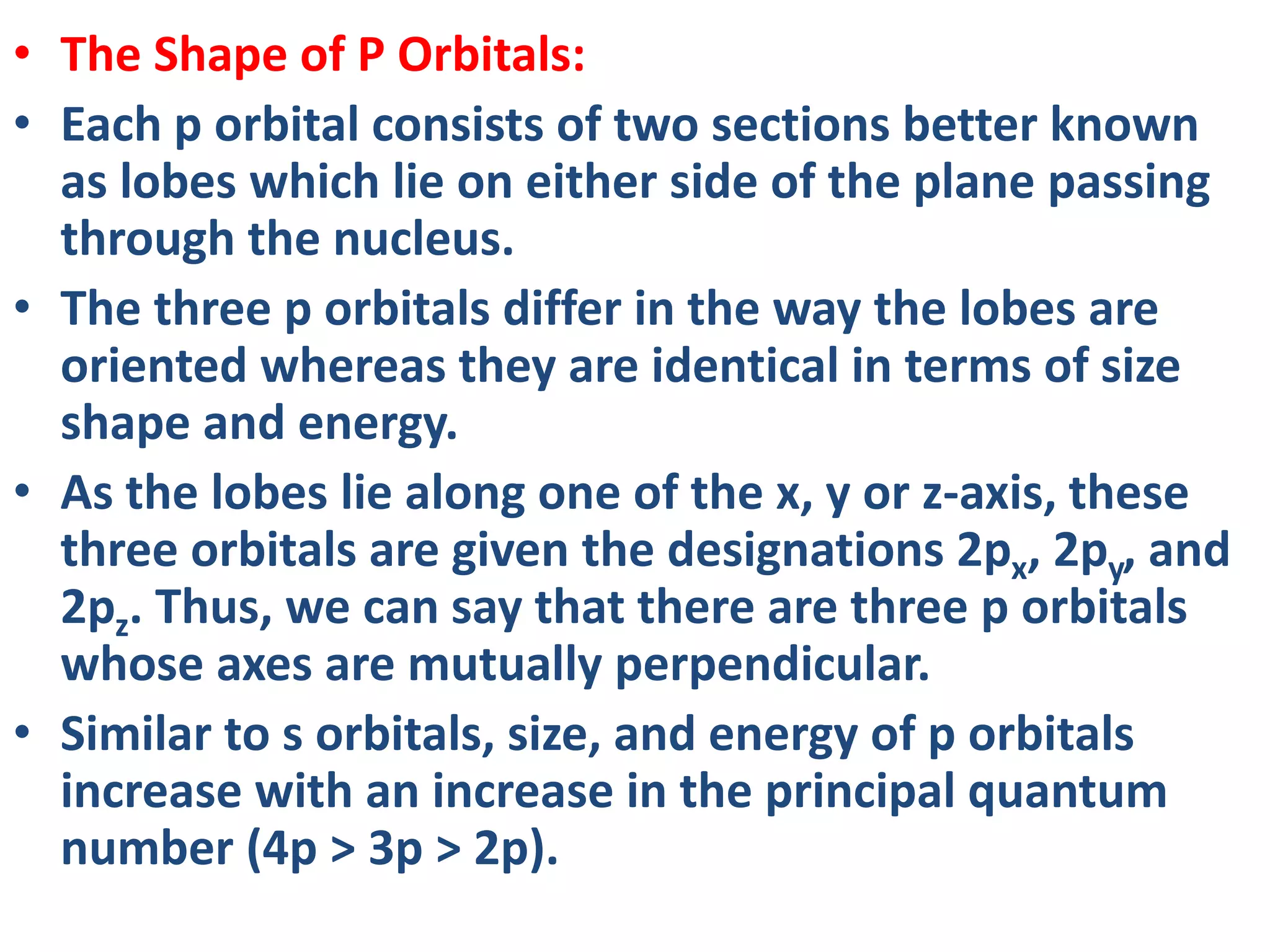
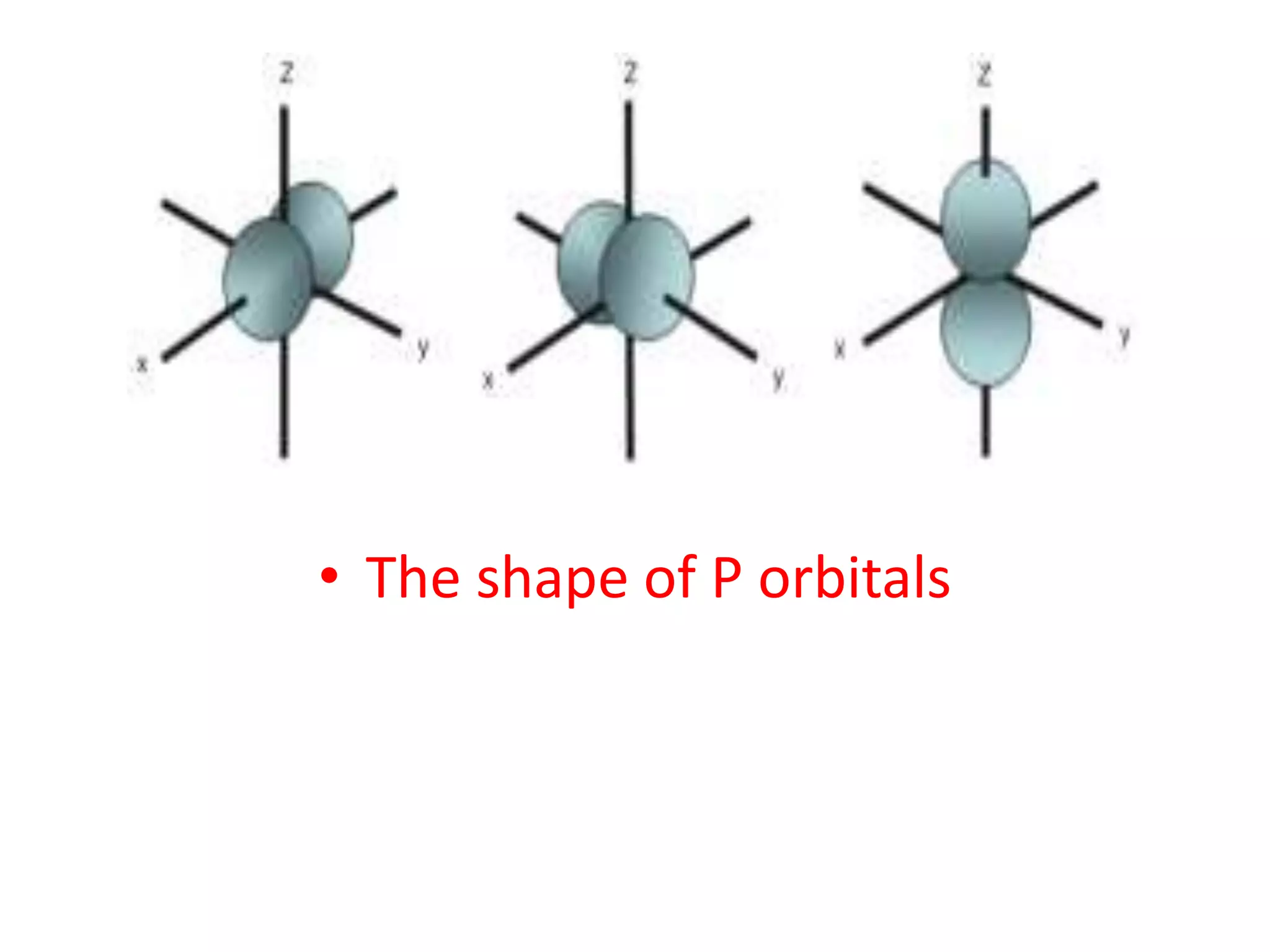

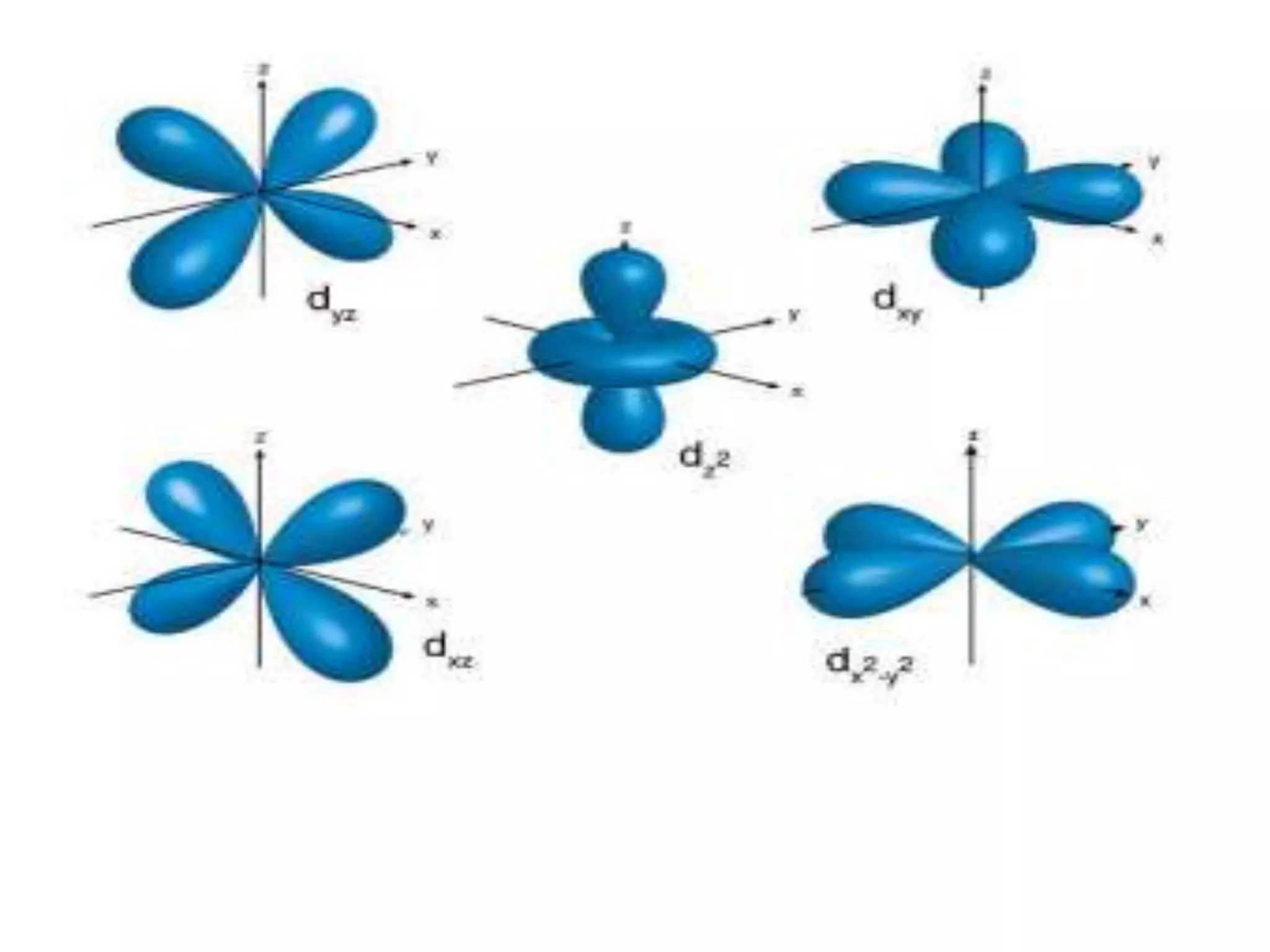
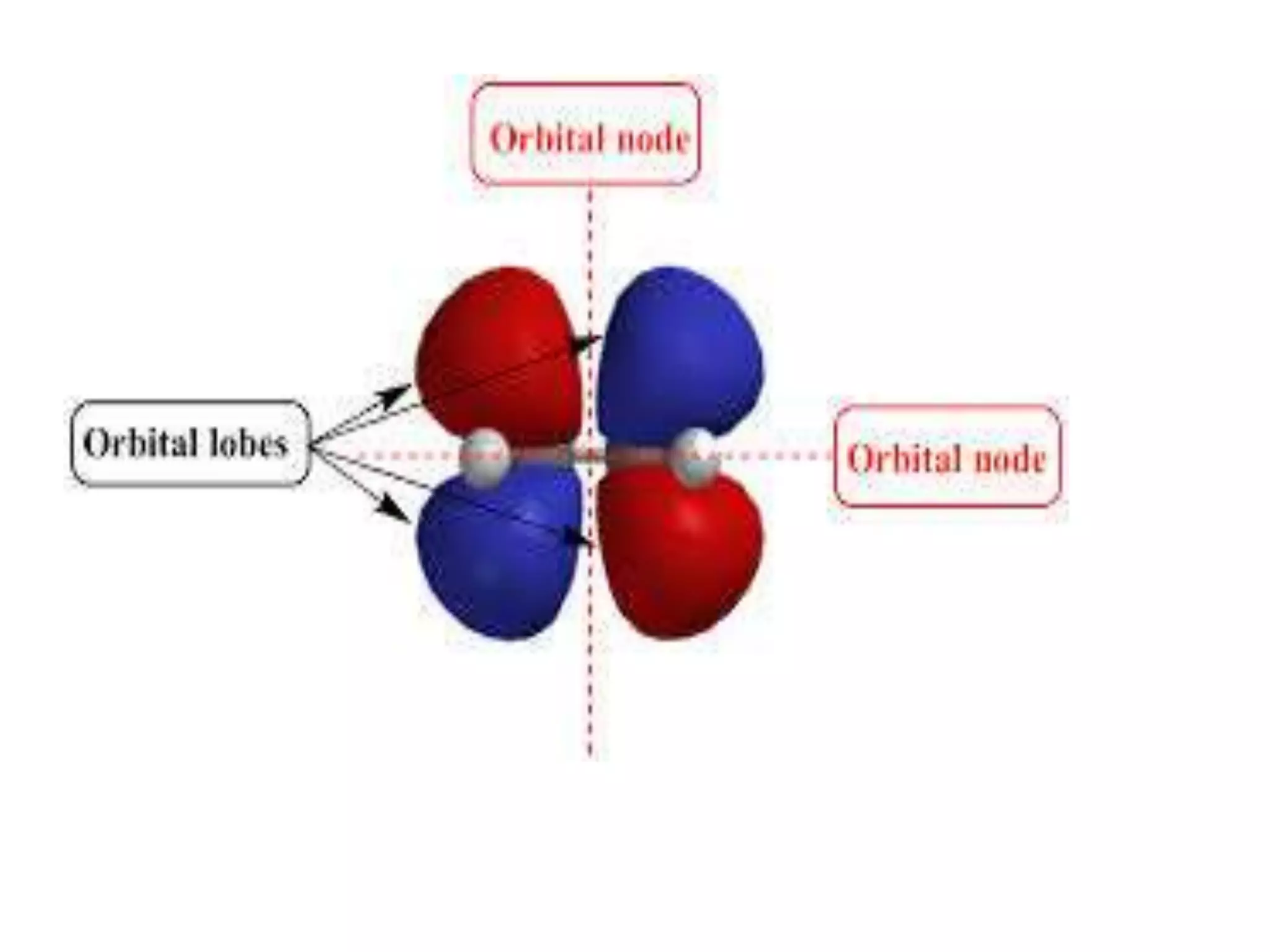
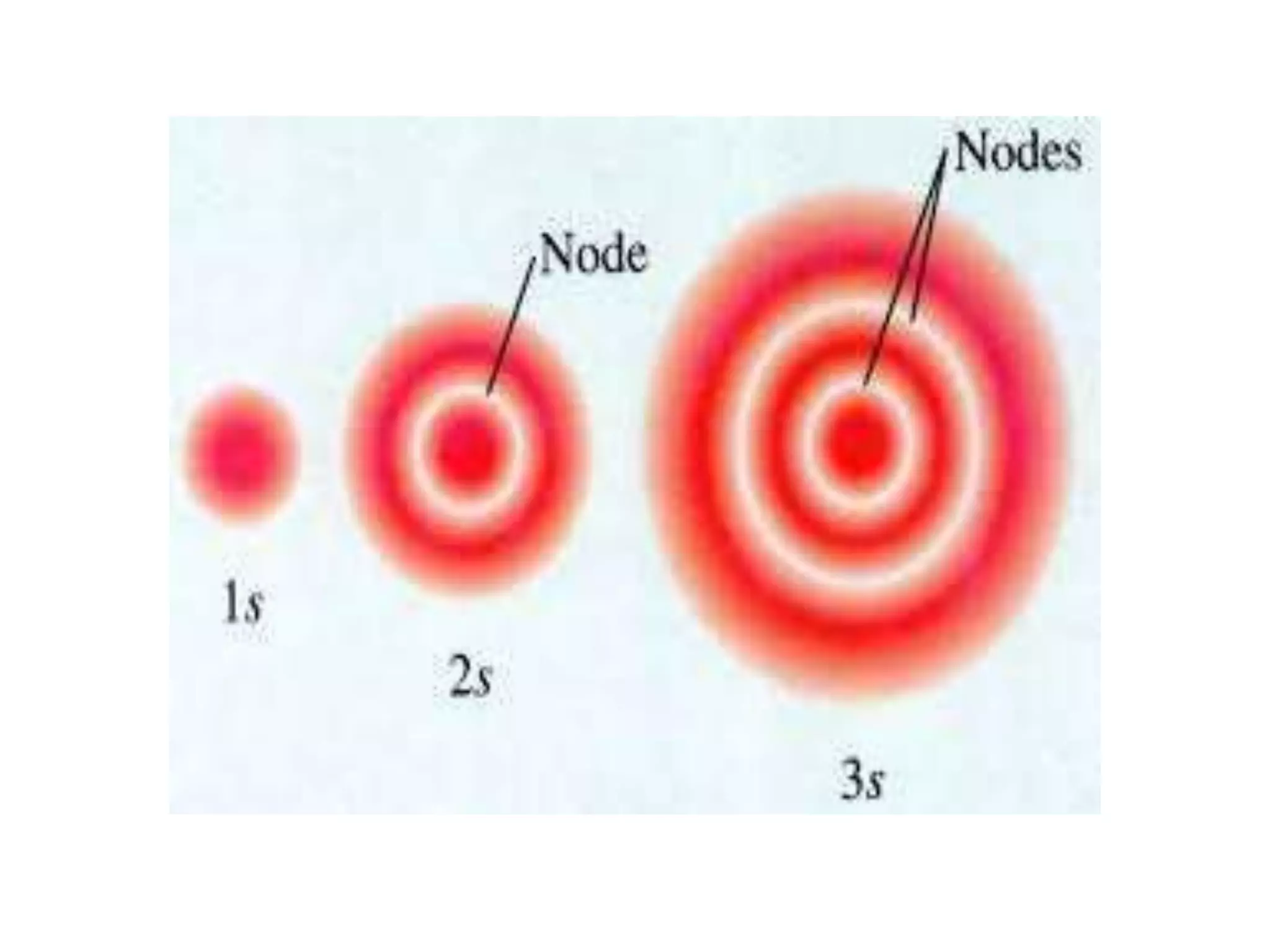
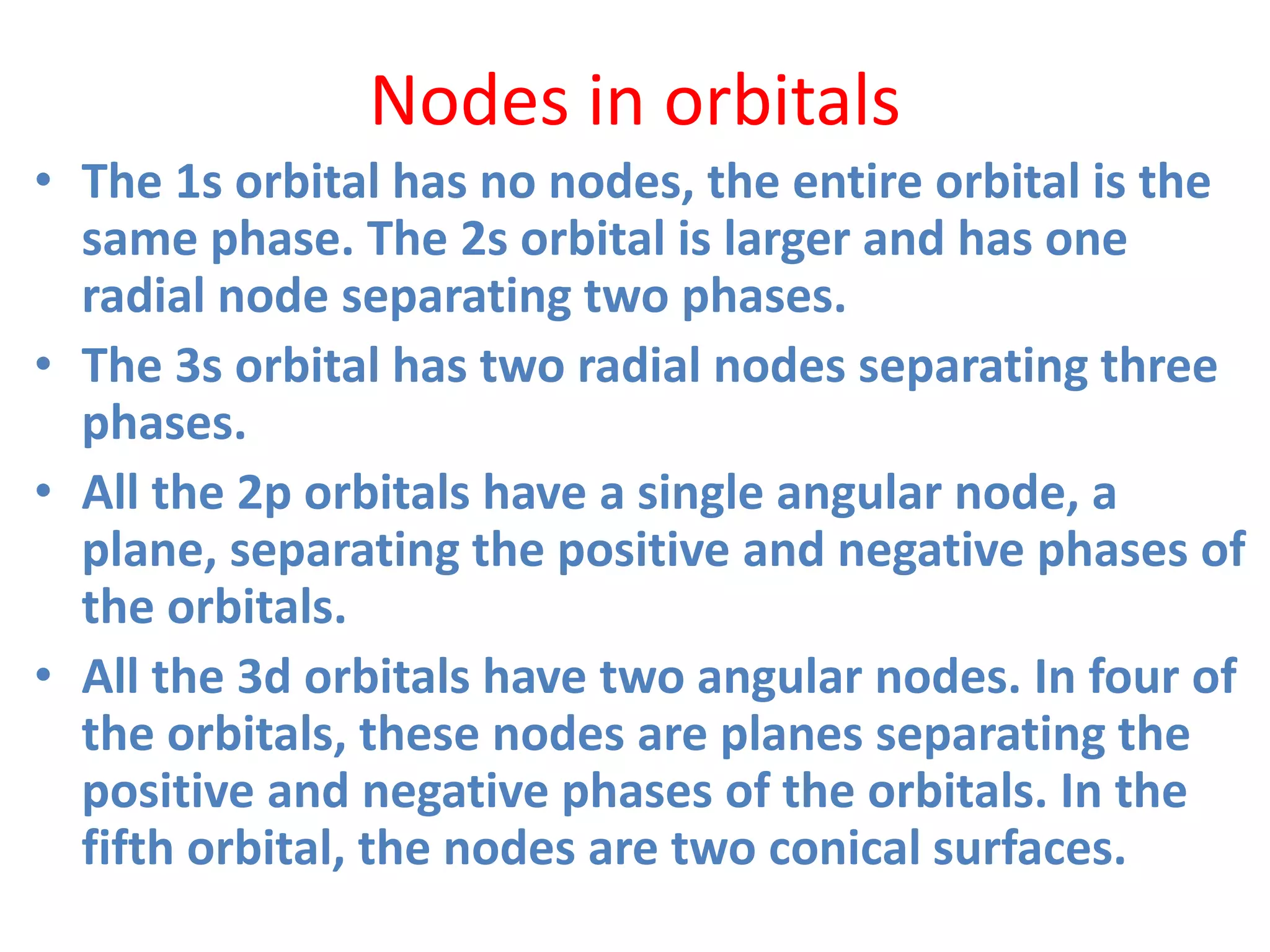

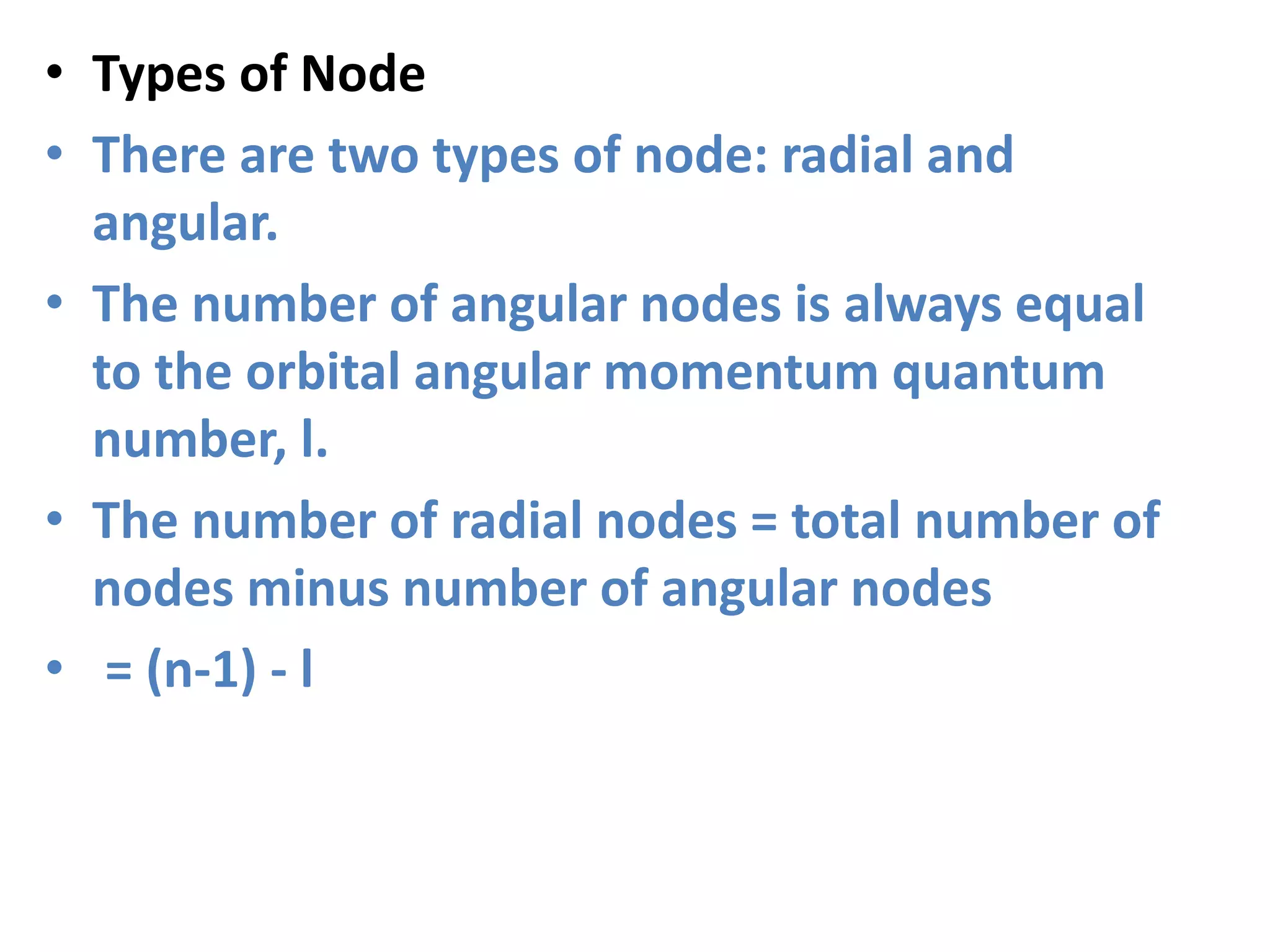
![Example
• Second Shell
In the second electron shell, the 2s orbital has
n=2 and l=0.
• The number of angular nodes = l = 0.
• The number of radial nodes
• = [(n-1) - l] = [1 - 0] = 1
• In the second electron shell,
The 2p orbital has n=2 and l=1.
The number of angular nodes = l = 1.
The number of radial nodes = [(n-1) - l] = [1 - 1] = 0
•](https://image.slidesharecdn.com/structureofatom-201129083435/75/Structure-of-atom-72-2048.jpg)
![• Third Shell
In the third electron shell, the 3s orbital has n=3
and l=0. The number of angular nodes = l = 0.
The number of radial nodes
• = [(n-1) - l] = [2 - 0] = 2
• In the third electron shell, the 3p orbital has n=3
and l=1. The number of angular nodes = l = 1.
The number of radial nodes
• = [(n-1) - l] = [2 - 1] = 1
• In the third electron shell, the 3d orbital has n=3
and l=2. The number of angular nodes = l = 2.
The number of radial nodes
• = [(n-1) - l] = [2 - 2] = 0](https://image.slidesharecdn.com/structureofatom-201129083435/75/Structure-of-atom-73-2048.jpg)