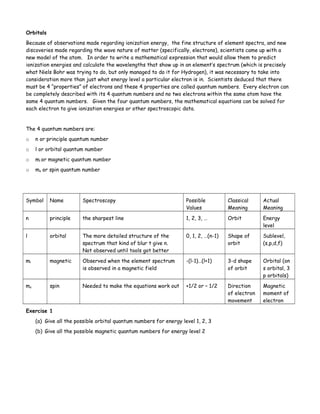
The document discusses orbitals and quantum numbers. It introduces the four quantum numbers - principal (n), azimuthal (l), magnetic (ml), and spin (ms) - that describe an electron's location and properties. The classical view of electrons orbiting the nucleus like planets gave way to the modern atomic model where electrons exist as probabilistic wave functions within orbitals. Orbitals are regions of high probability of finding an electron and come in s, p, d, and f shapes depending on the quantum numbers. Electron configurations use these orbitals to describe the arrangement of electrons in atoms and predict properties.



![Now that you know what an orbital is, you need to know how to use the orbital to describe the electron
structure of elements. There are two ways to do this:
(1) electron configurations
a. Standard: 1s2
2s2
2p6
3s2
3p6
4s2
3d10
…
b. More detailed: 1s2
2s2
2px
2
2py
2
2pz
2
3s2
3px
2
3py
2
3pz
2
4s2
3dz2
2
3dx2-y2
2
3dxy
2
3dxz
2
3dyz
2
…
i. Since all p orbitals are degenerate (have the same energy) it doesn’t matter which
one you put electrons in first, but see below for Hund’s rule
ii. Same goes for d orbitals and f orbitals
c. Noble Gas: [Ne] 3s2
…
(2) orbital diagrams (arrows)
Aufbau described the order that electrons fill the orbitals: from lowest to highest energy. It may seem a
little odd, but the 4s orbital is actually lower in energy than the d orbitals in the 3rd
energy level. To
remember the order you can use the following :
In an atom the orbitals are filled in order of increasing energy, starting from 1s.
An aid to remembering the order in which orbitals are filled is to write them down in columns as shown.
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f
6s 6p 6d
7s 7p
The order of filling is then given by drawing diagonal lines through the symbols.
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5s 5p 5d 5f
6s 6p 6d
7s 7p
Exercise 6
Write out the complete order in which orbitals are filled:](https://image.slidesharecdn.com/orbitalshlnotes-140707065510-phpapp01/85/Orbitals-hl-5-320.jpg)
![One other rule you must know: Hund’s Rule
Degenerate orbitals (of the same energy) are occupied by one electron before any orbital is occupied by a
second electron and all electrons in singly occupied orbitals must have the same spin.
Exercise 7
On a separate sheet of paper write the electron configuration and orbital diagrams for elements 3 to 10.
Exercise 8
Give the quantum numbers for all 8 of oxygen’s electrons.
Exercise 9
An electron has quantum numbers( n, l, ml, ms): 3, 0, 0, +1/2
(a) In what energy level is the electron located?
(b) In what subshell is the electron located?
(c) What is the shape of this subshell?
An electron has quantum numbers: 3, 1, -1, -1/2, draw it’s orbital diagram.
Exercise 8
(a) Write the electron configuration for Na.
(b) Explain why the electron configuration for Na can be written [Ne] 3s1](https://image.slidesharecdn.com/orbitalshlnotes-140707065510-phpapp01/85/Orbitals-hl-6-320.jpg)
