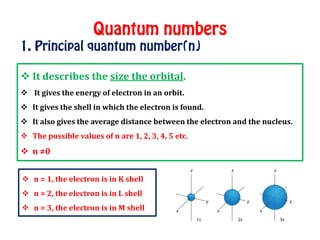
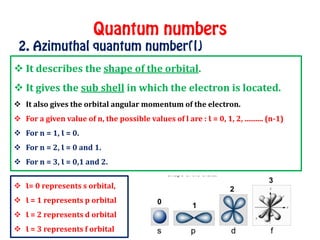
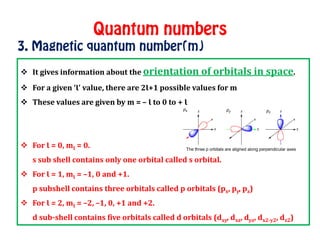
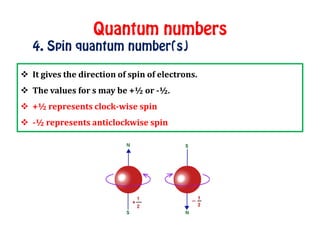
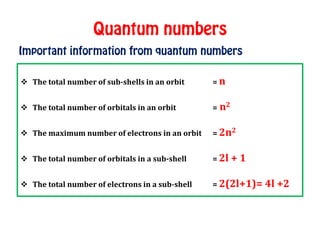
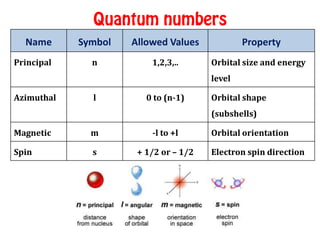
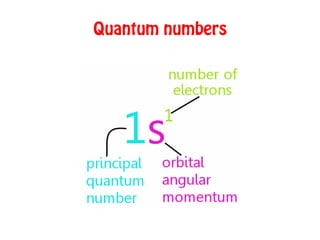
The document discusses the four quantum numbers that describe electrons in atoms: principal quantum number (n), azimuthal/angular momentum quantum number (l), magnetic quantum number (m), and spin quantum number (s). N determines the shell and average distance from the nucleus. L determines the subshell and shape. M gives orbital orientation. S indicates electron spin direction as clockwise or counterclockwise. Each number has specific allowed values and properties that provide the electron's precise location.