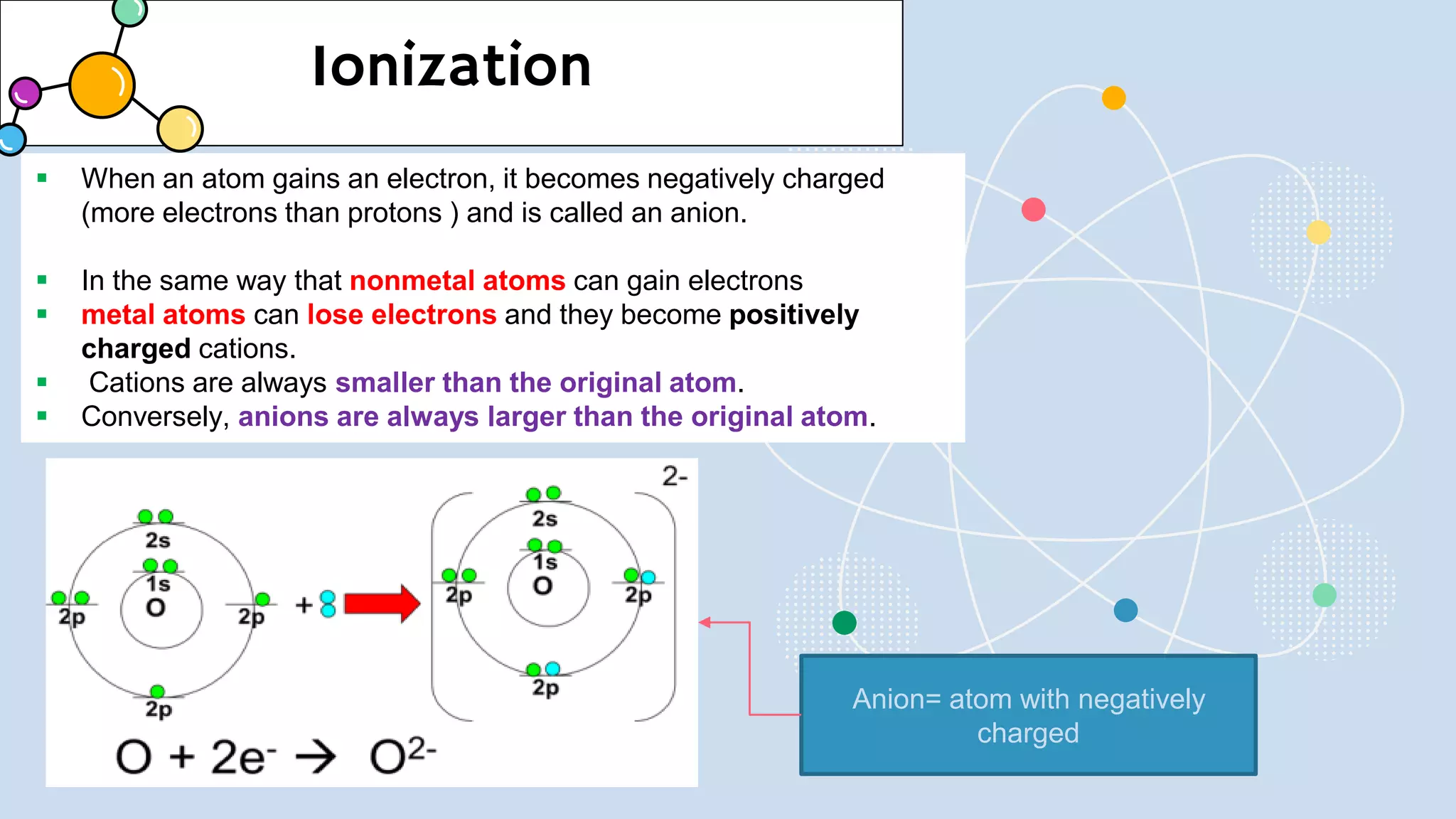
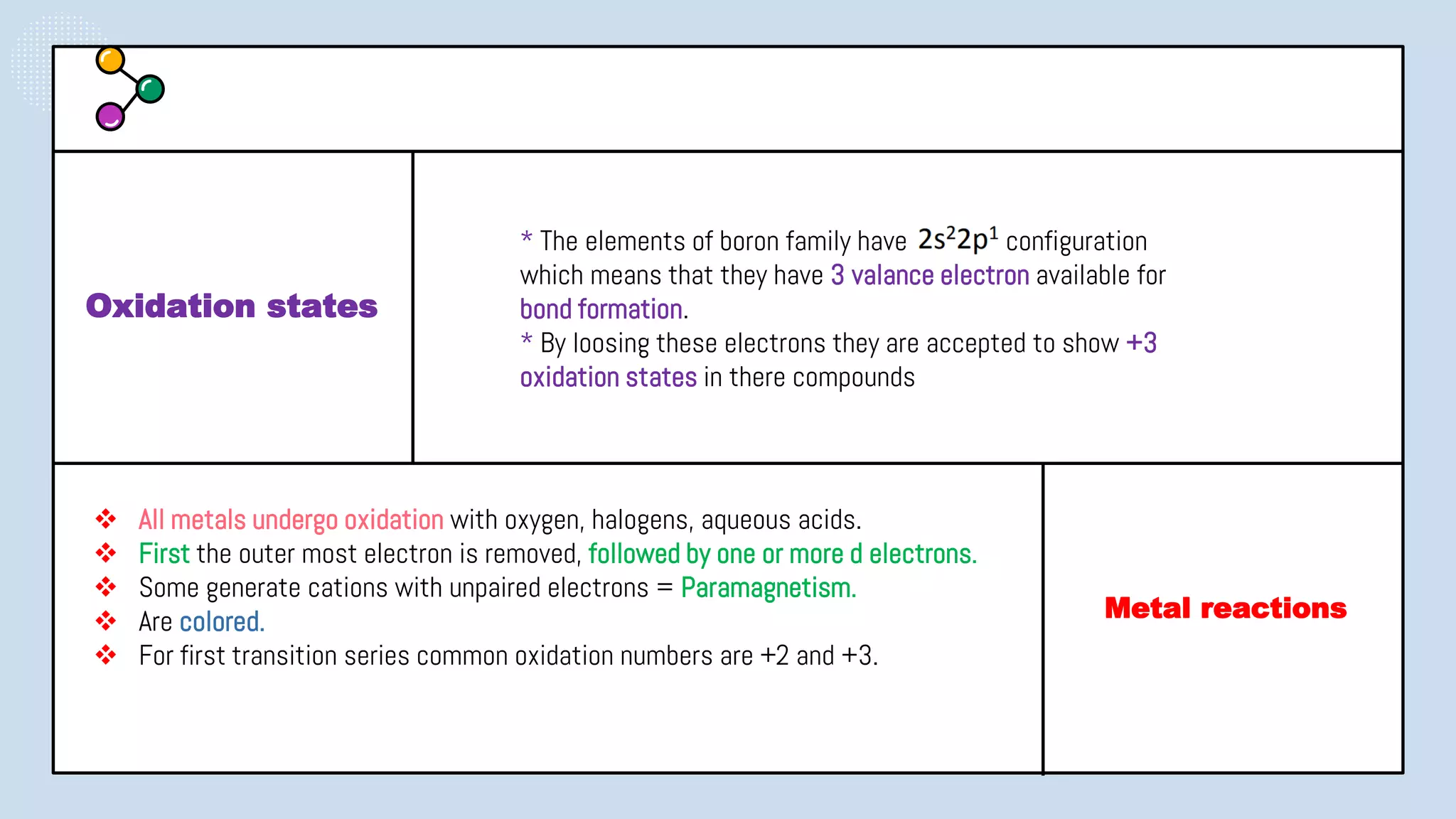
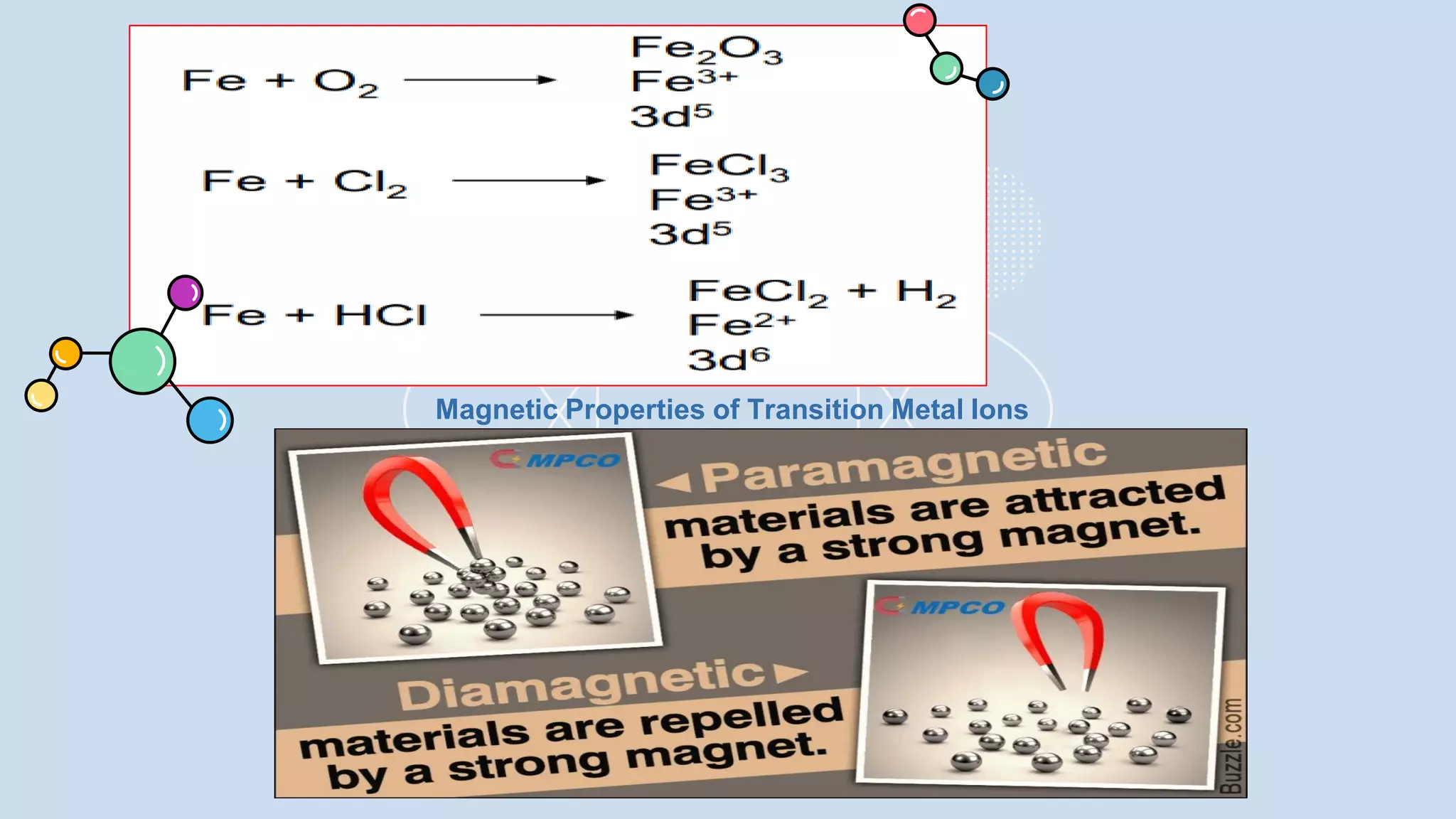
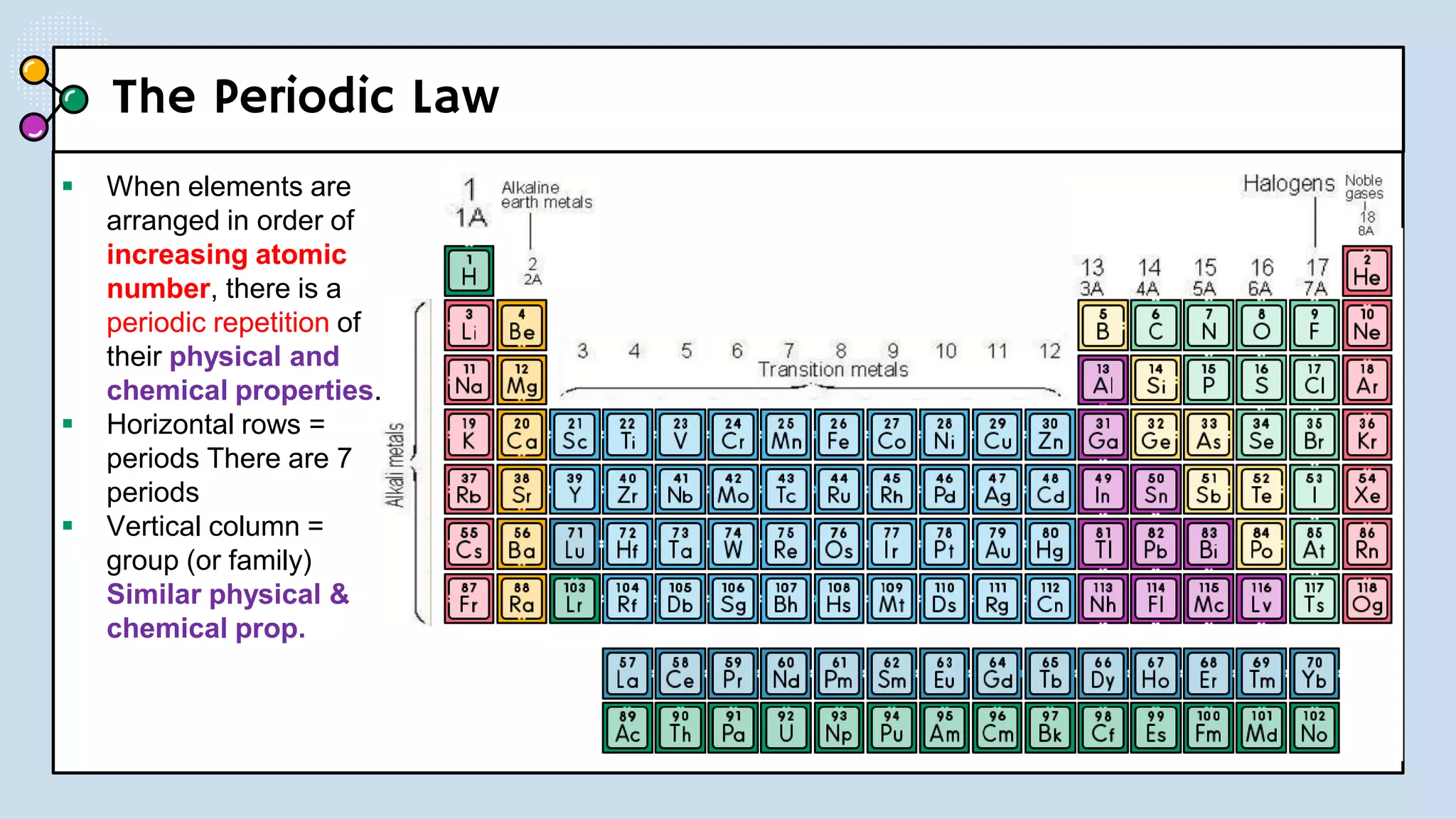
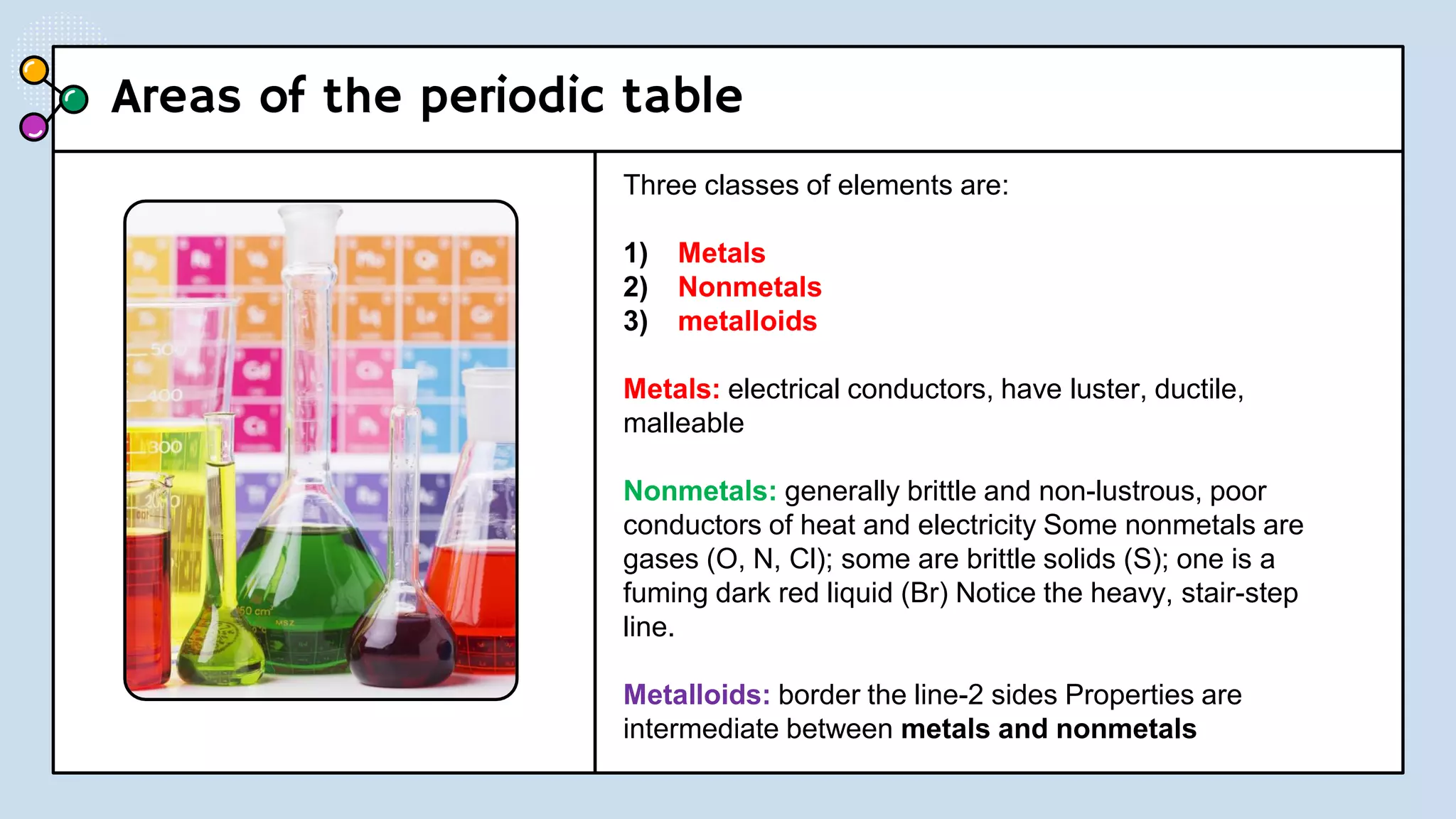
This document provides a 3-paragraph summary of the contents of an inorganic pharmaceutical chemistry course titled "Atomic and Molecular Structure/Complexation". The course will cover 9 topics including atomic and molecular structure, electrolytes, essential and trace ions, non-essential ions, gastrointestinal agents, antacids, protective adsorbents, radiopharmaceutical preparations, and radio opaque and contrast media. It will examine the fundamental atomic structure including protons, neutrons, electrons, isotopes and quantum mechanics. The course will also cover electronic configurations, orbitals, quantum numbers, ionization, periodic trends, and transition metal ions. The instructor is Hayder R. Fadhil and the course is listed as the 1st semester, 3

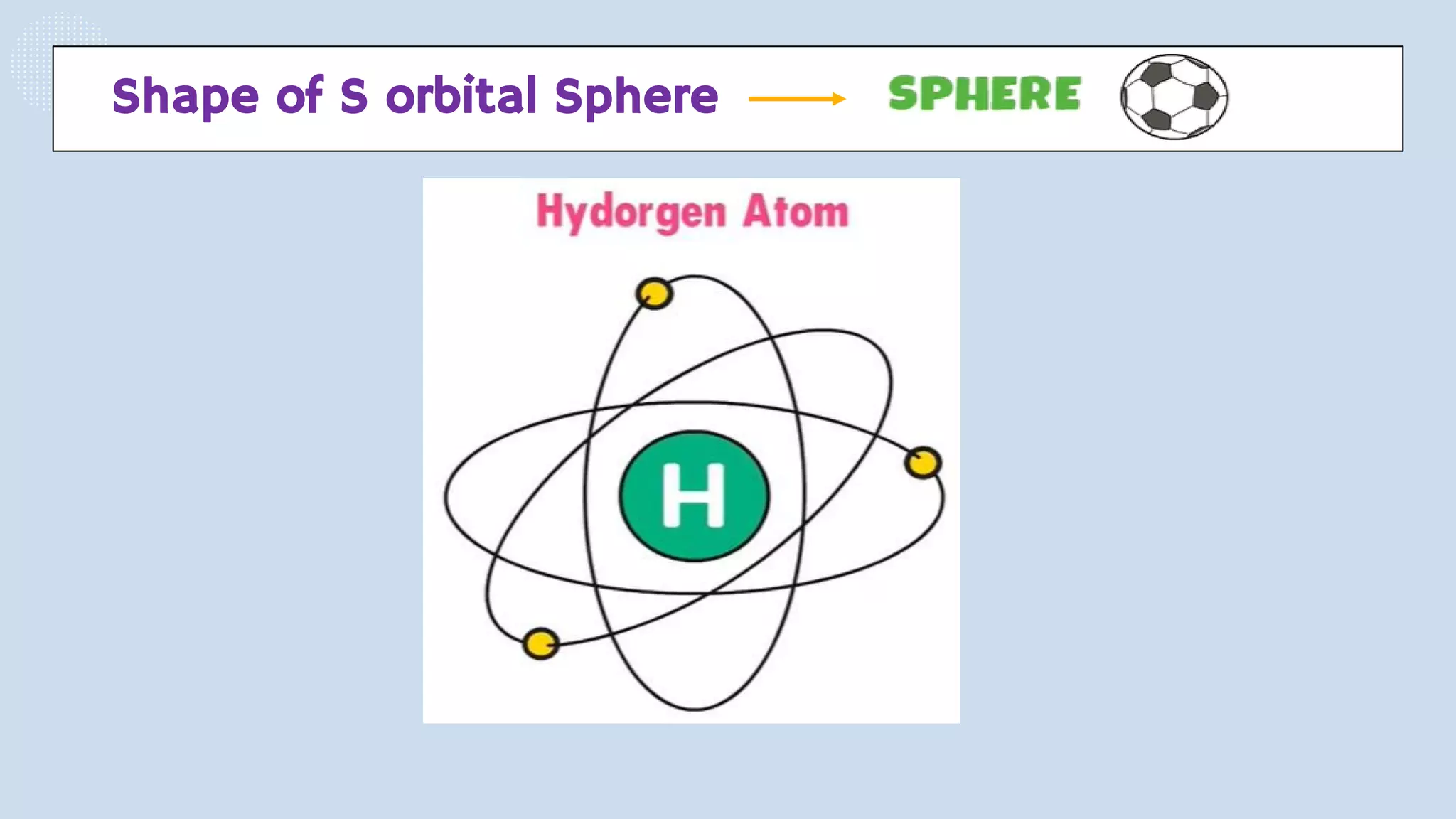
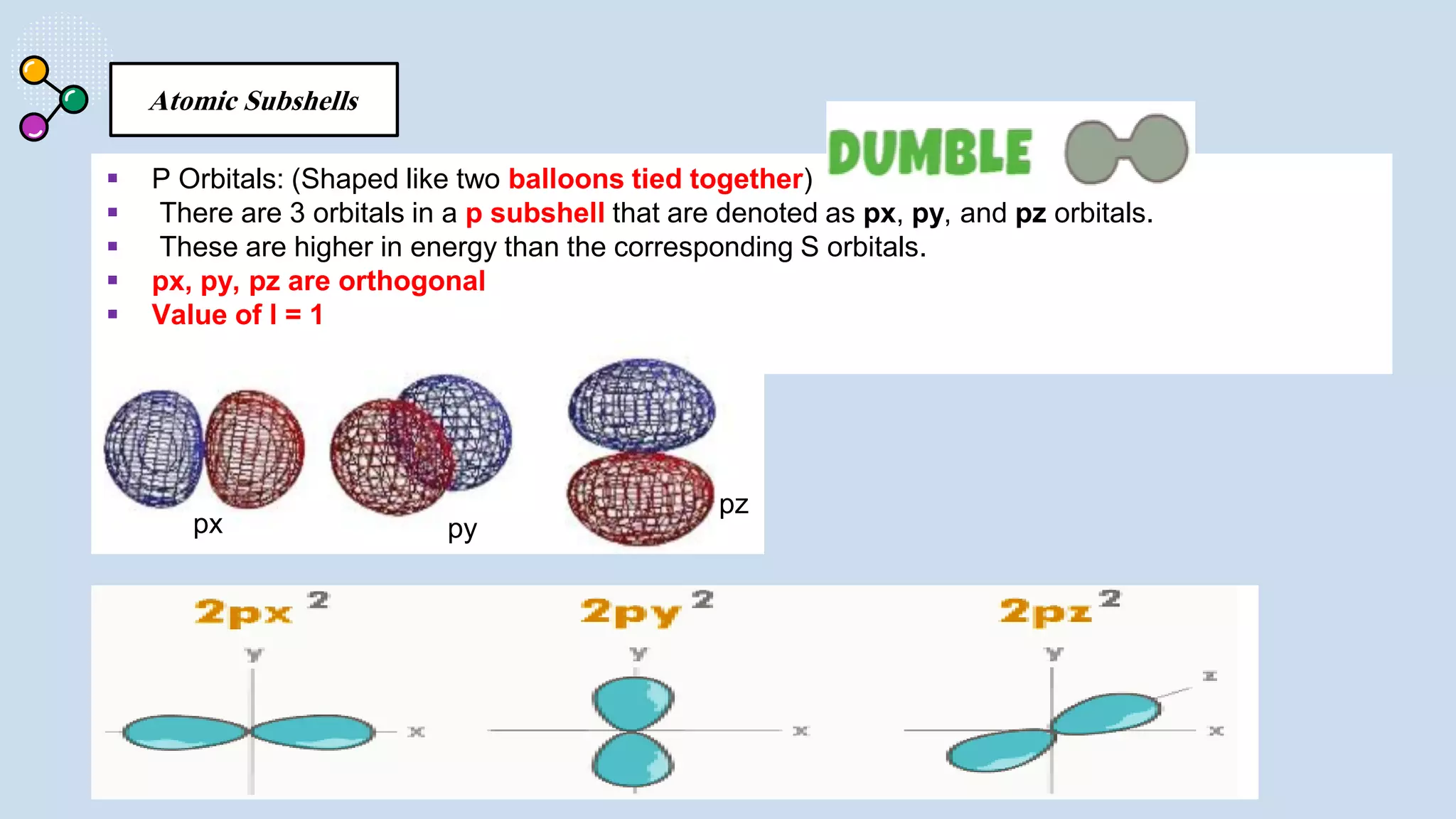
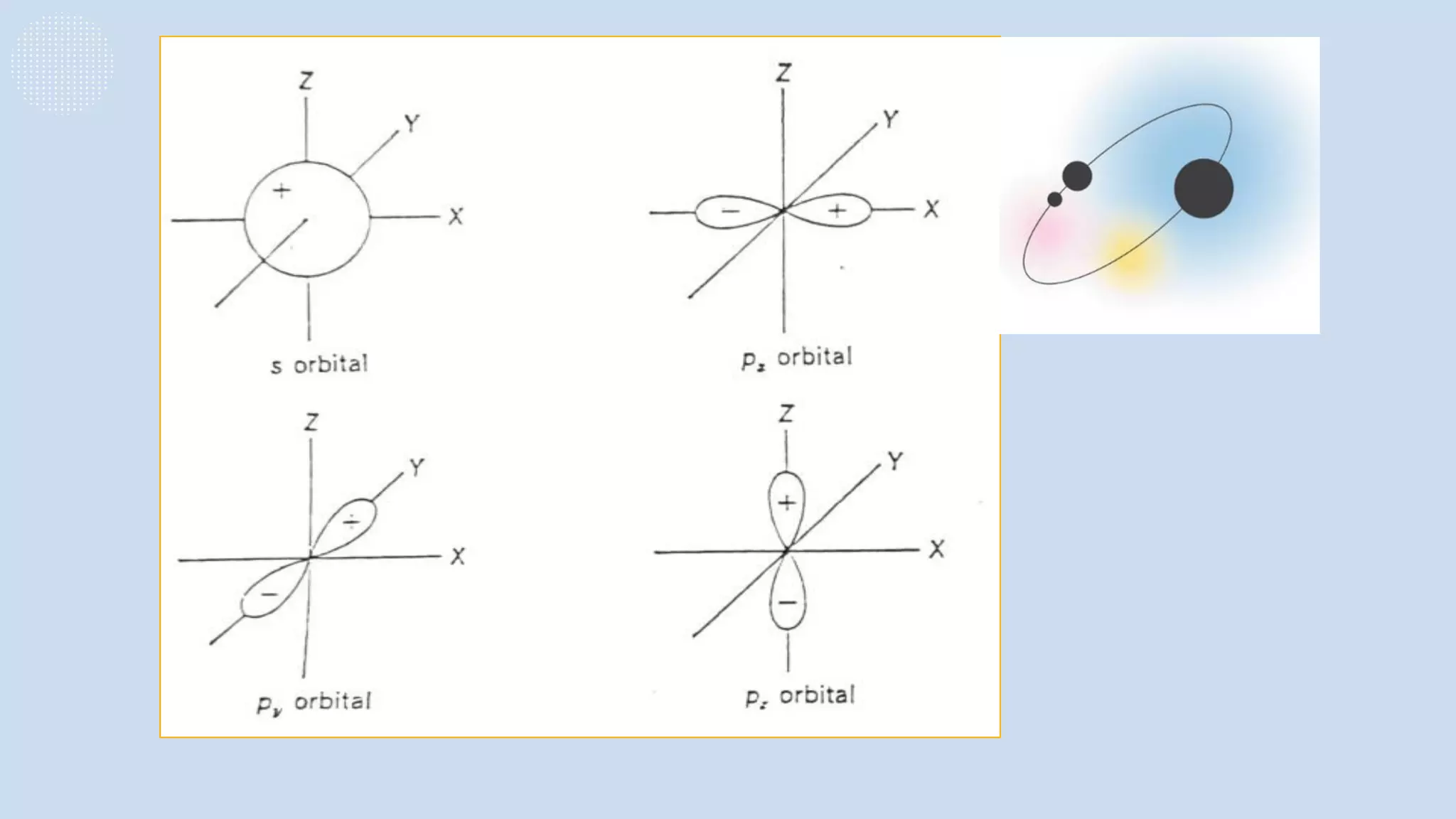
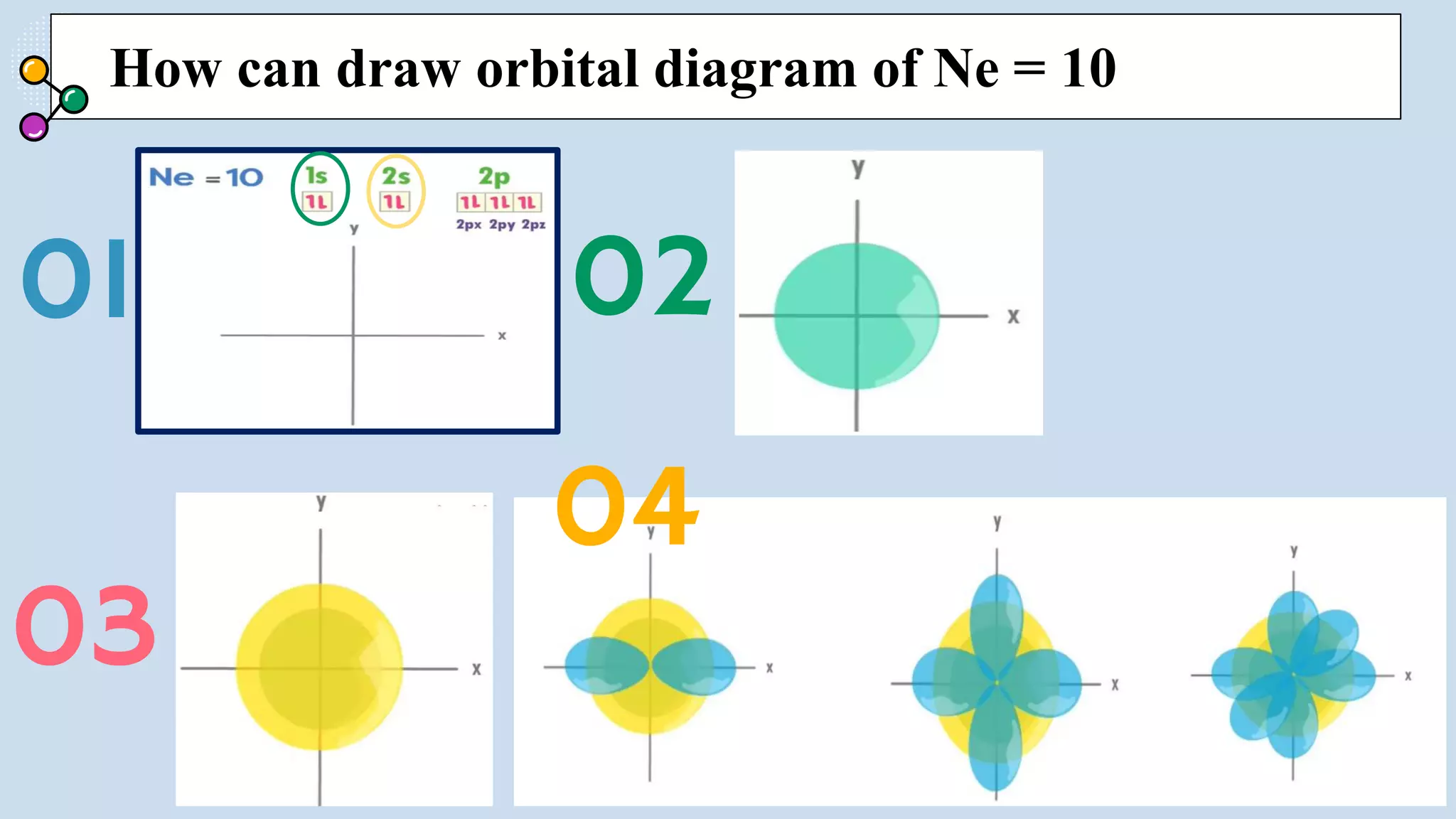
![The Quantum Mechanical Model
A quantum is the amount of energy needed to move
from one energy level to another.
Since the energy of an atom is never “in-between” there
must be a quantum leap in Energy.
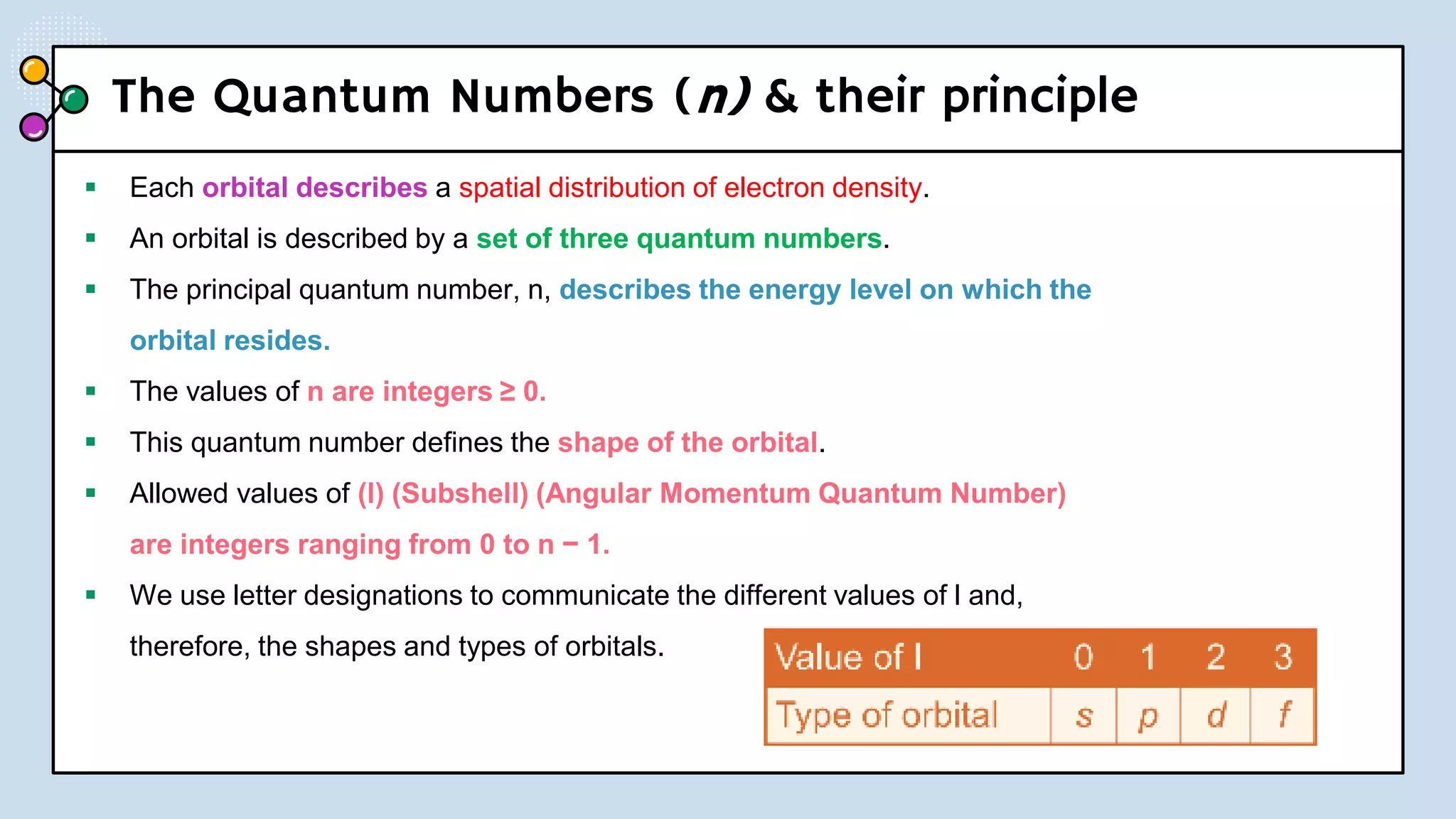
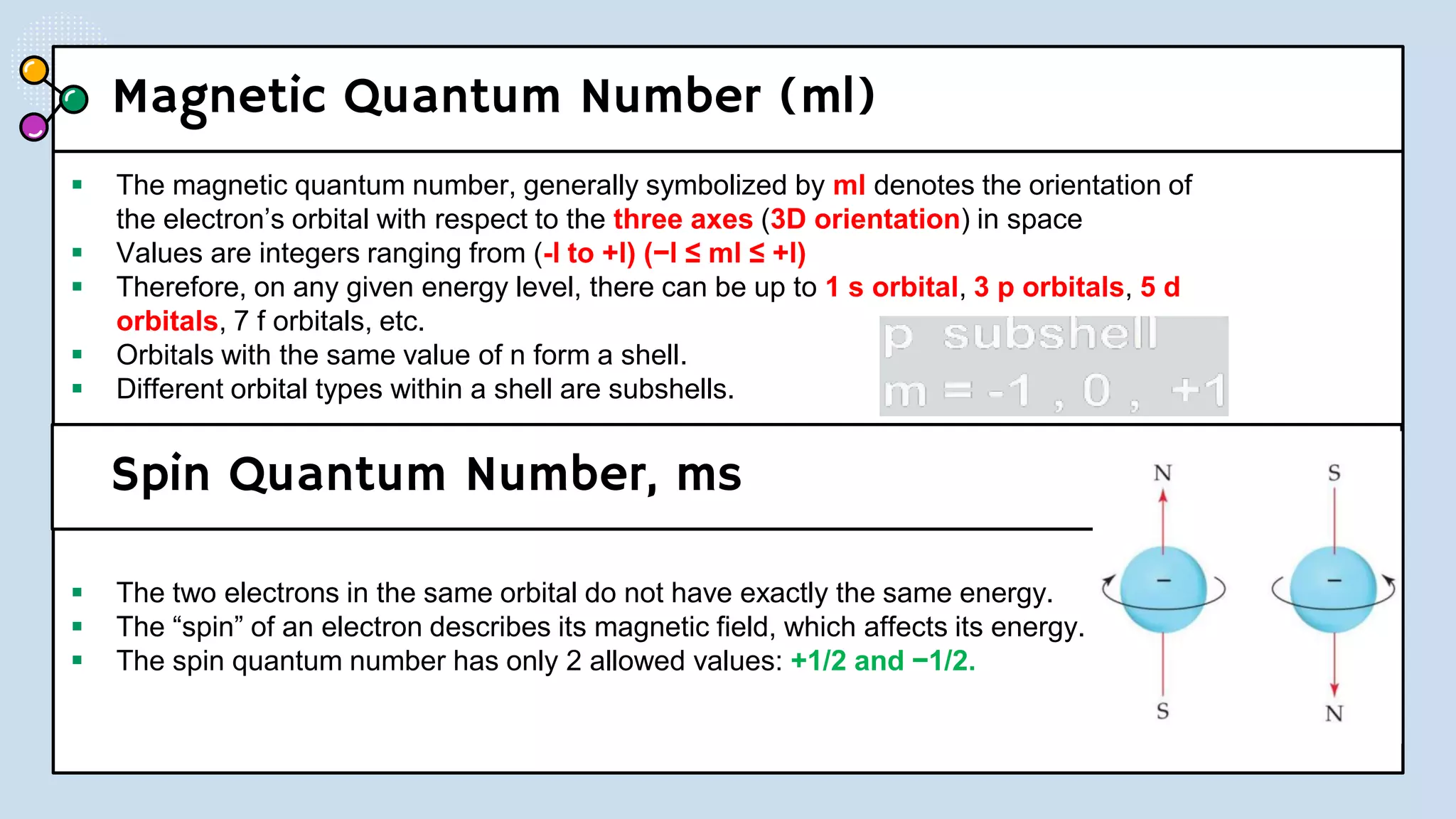
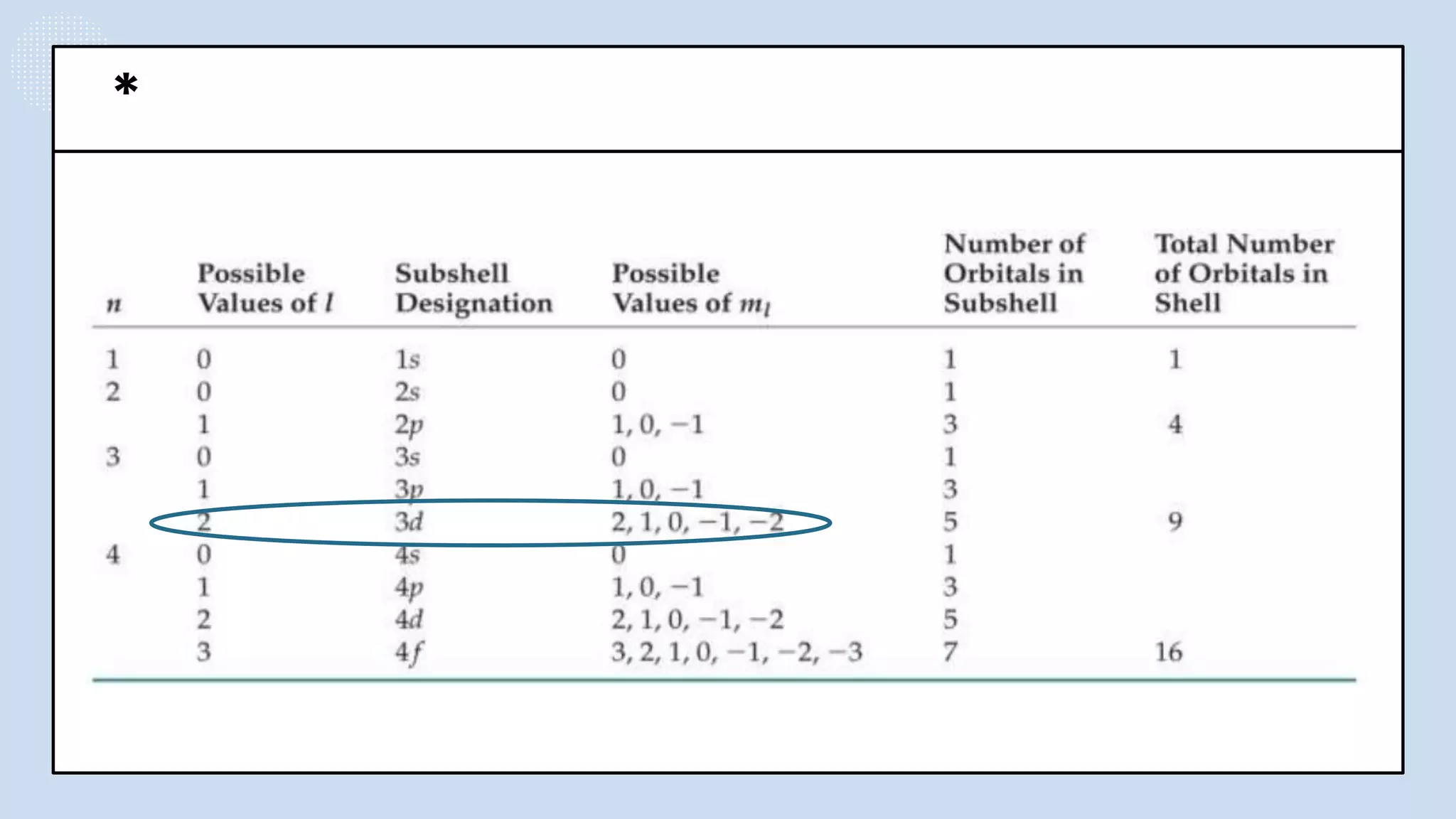
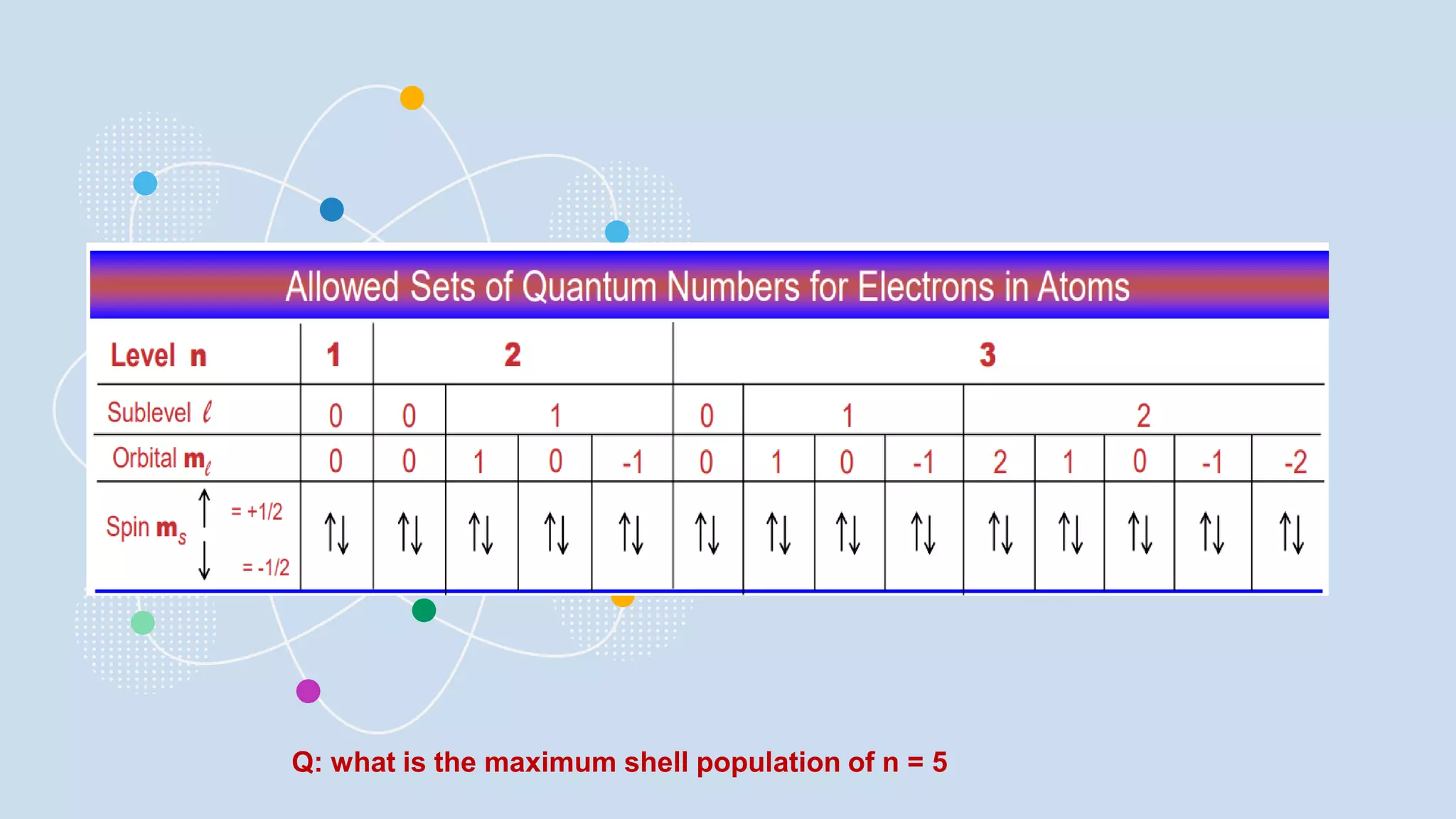
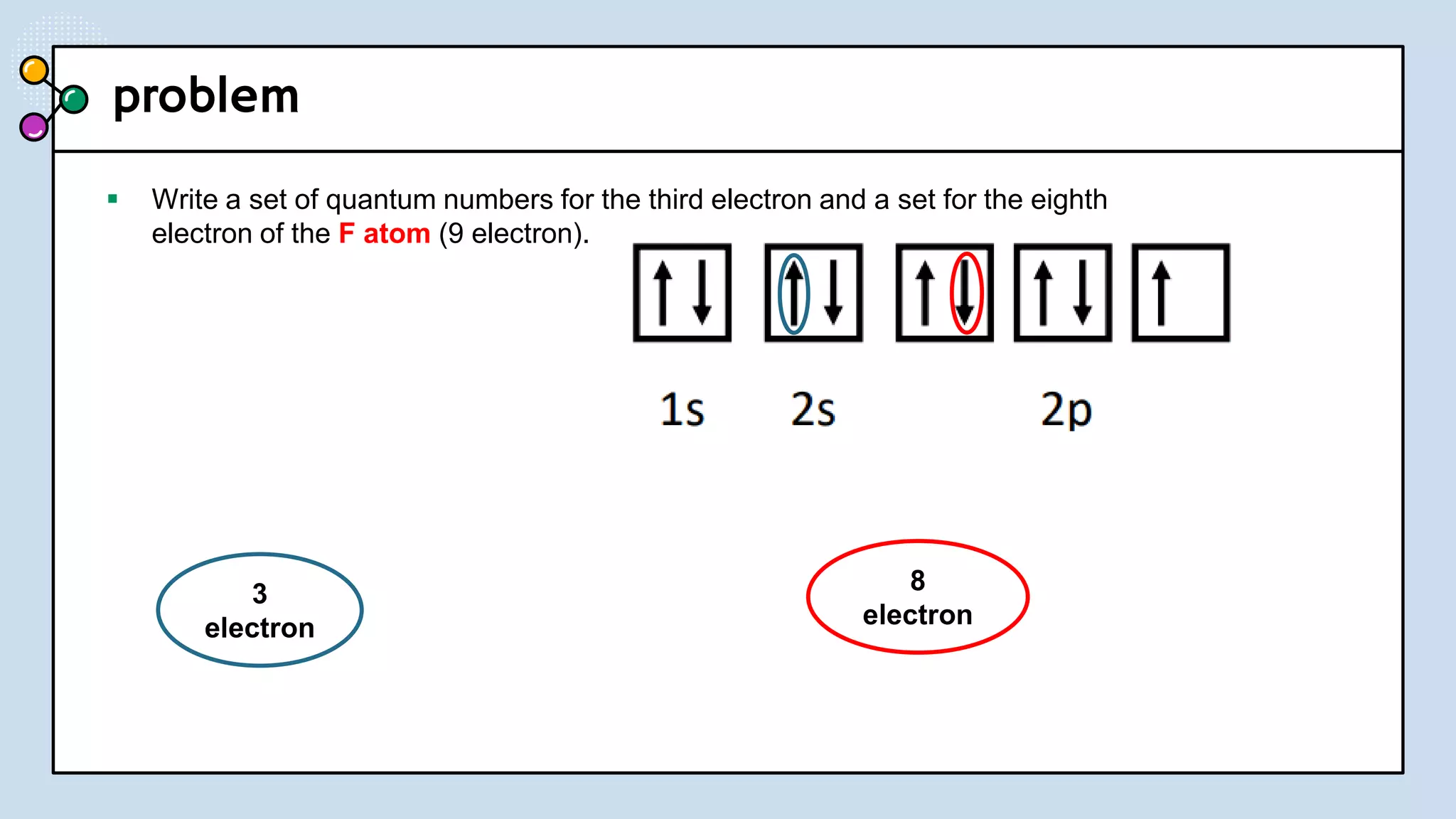
NOTES: Pauli Exclusion Principle No two electrons in
an atom can have the same four quantum numbers.
[energy level (n)(shells) , subshells (l), orbital (ml) and
spin (ms)].
- No two electrons in the same atom can have exactly the
same energy.
- For example, no two electrons in the same atom can have
identical sets of quantum numbers.](https://image.slidesharecdn.com/lec-231003091649-96cec002/75/ATOMIC-AND-MOLECULAR-STRUCTURE-16-2048.jpg)