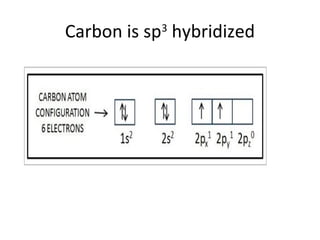
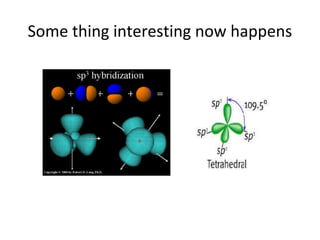
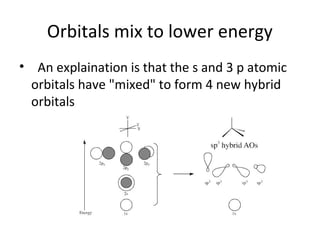
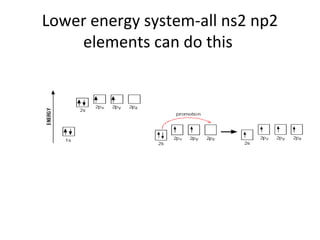
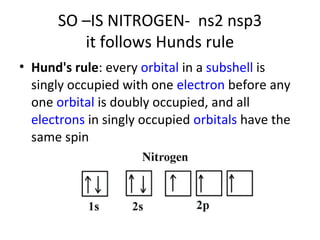
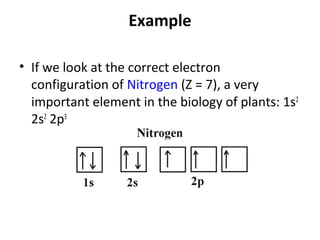
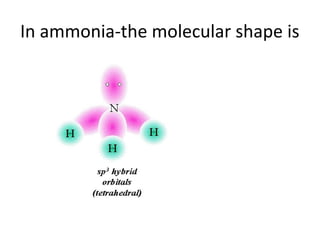
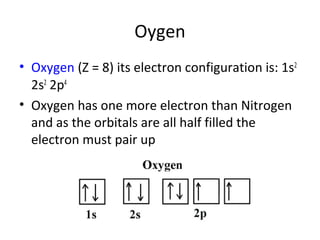
In 1934, Schrödinger lectured at Princeton University and was offered a permanent position, but he did not accept it. The document then provides an agenda for a lecture on Schrödinger and topics in quantum theory, including his famous cat experiment, quantum numbers, and principles like Hund's rule and the Pauli exclusion principle. It concludes by mentioning seeing Dr. Poget after class and that much of modern knowledge is based on quantum theory foundations.


















![Period 4:
• Chromium: Z:24 [Ar] 3d54s1
• Copper: Z:27 [Ar] 3d104s1](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-38-320.jpg)
![Chromium:
• Chromium: Z:24 [Ar] 3d54s1](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-39-320.jpg)
![Copper
• Copper: Z:27 [Ar] 3d104s1
• Example: In the following configuration,
• Cu: [Ar]4s23d9, copper's d shell is just one away
from stability, and therefore, one electron
from the s shell jumps into the d shell:
[Ar]4s13d10. This way, the d shell is full, and is
therefore stable, and the s shell is half full,
and is also stable.](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-40-320.jpg)
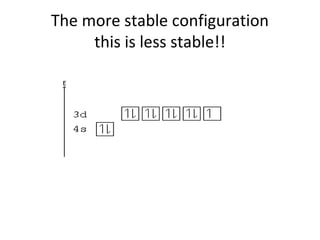
![Most stable configurations####
• Chromium has a configuration of [Ar]4s13d5,
• The stability rule applies to atoms in the same
group as chromium and copper.
• If one of these atoms has been ionized, that is,
it loses an electron, it will come from the s
orbital rather than the d orbital.
• the configuration of Cu+ is [Ar]4s03d10.](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-42-320.jpg)
![Loss of outer electrons
• If one of these atoms has been ionized, that is,
it loses an electron, it will come from the s
orbital rather than the d orbital. For instance,
the configuration of Cu+ is [Ar]4s03d10. If more
electrons are removed, they will come from
the d orbital](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-43-320.jpg)

![Period 5:
• Niobium: Z:41 [Kr] 5s1 4d4
• Molybdenum: Z:42 [Kr] 5s1 4d5
• Ruthenium: Z:44 [Kr] 5s1 4d7
• Rhodium: Z:45 [Kr] 5s1 4d8
• Palladium: Z:46 [Kr] 4d10](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-47-320.jpg)





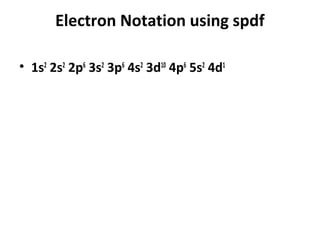
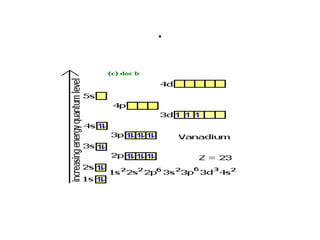
![Electron Notation using spdf
• Example
• Vanadium (V, Z=23) lies in the transition metals
at the four period in the fifth group. The noble
gas before it is argon, (Ar, Z=18) and knowing
that vanadium has filled those orbitals before it,
we will use argon as our reference noble gas. We
denote the noble gas in the configuration as the
symbol, E, in brackets: [E] configuration:
• Vanadium, V: [Ar] 4s2 3d3](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-59-320.jpg)
![Electron Configurations of Ions
• Writing electron configurations for ions,
whether it be cation or anion, is basically
exactly the same as writing them for normal
elements. All the same rules apply, except you
must take into account the gained or lost
electrons. For instance, when Potassium (K)
loses an electron it becomes K+ and has the
noble gas configuration of [Ar].](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-61-320.jpg)
![electron configuration
• K ([Ar]4s1) --> K+([Ar]) + e-
Therefore, the electron configuration for the
K+ ion is simply [Ar]
• When an atom, such as Chlorine (Cl) gains an
electron, it becomes Cl- and also has the
electron configuration of [Ar].
• Cl ([Ne]3s23p5) + e- --> Cl- ([Ar])
Yet again, the electron configuration is [Ar]](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-62-320.jpg)
![• For more complex ionic electron configurations, such
as an ion from the transition metals, the answer isn't
always a noble gas. Take Iron (Fe). The most common
irons for Iron are Fe2+ and Fe3+. Lets focus on Fe2+.
• Fe ([Ar]3d64s2) --> Fe2+ ([Ar]3d6) - 2e-
Here Iron loses two electrons. So thats two less electrons to fill orbitals. When you backtrack two electrons in Fe's original electron configuration you
[Ar]3d6 as Fe2+'s new configuration
get
• When writing the electron configuration for ions, treat
it like any normal element. Just remember to simply
add or subtract the gained or lost electrons when
filling out shells.](https://image.slidesharecdn.com/chapter7schrodinger2003-121121145219-phpapp02/85/Chapter-7-schrodinger2003-63-320.jpg)