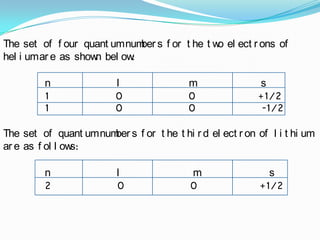
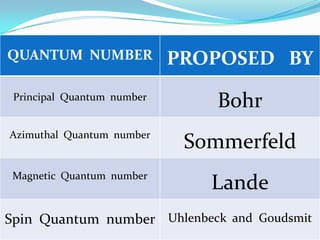
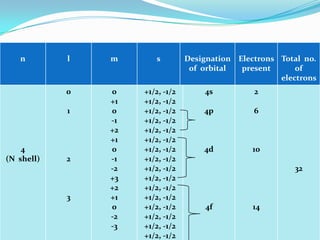
The four quantum numbers - principal (n), azimuthal (l), magnetic (m), and spin (s) - are used to completely characterize electrons in an atom. The principal quantum number indicates the electron's energy level. The azimuthal number denotes the subshell and shape of its orbital. The magnetic number determines the number of orbitals in a subshell. The spin number arises from the electron's intrinsic spin and can have values of ±1/2. Together, the four quantum numbers uniquely identify each electron in an atom.


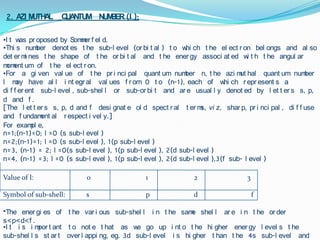


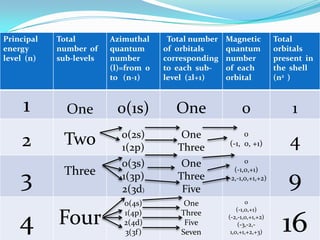
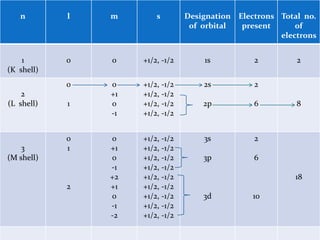
![For a given value of the principal quantum number n, the azimuthal quantum number l may have all integral values from 0 to (n-1), each of which represents a different sub-level, sub-shell or sub-orbit and are usually denoted by letters s, p, d and f.[The letters s, p, d and f designate old spectral terms, viz, sharp, principal, diffuse and fundamental respectively.]For example, n=1;(n-1)=0; l=0 (s sub-level)n=2;(n-1)=1; l=0 (s sub-level), 1(p sub-level) n=3, (n-1) = 2; l=0(s sub-level), 1(p sub-level), 2(d sub-level)n=4, (n-1) =3; l=0 (s sub-level), 1(p sub-level), 2(d sub-level),3(f sub- level)Value of l: 0 1 2 3 Symbol of sub-shell: s p d fThe energies of the various sub-shell in the same shell are in the order s<p<d<f.](https://image.slidesharecdn.com/quantumnumbers-100507003716-phpapp01/85/Quantum-numbers-7-320.jpg)