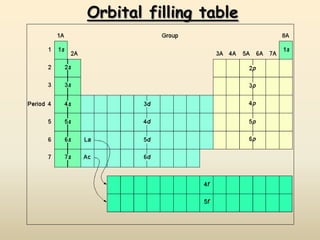
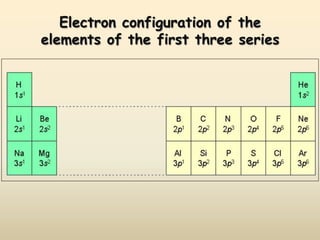
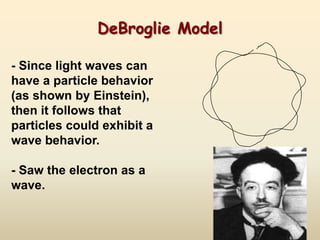
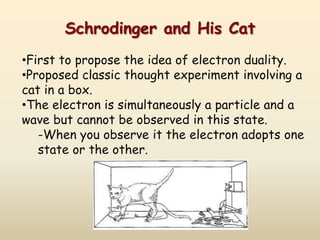
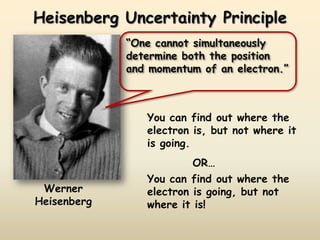
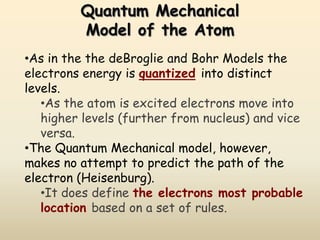
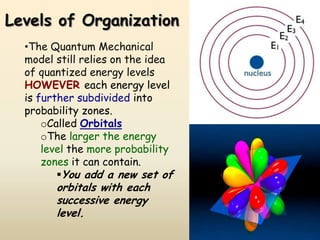
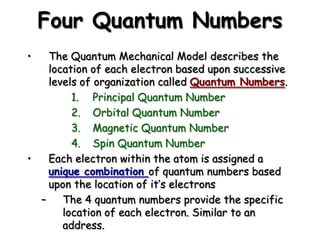
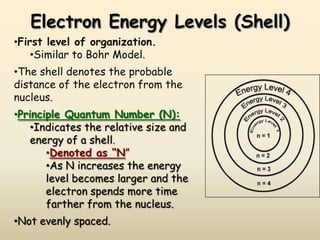
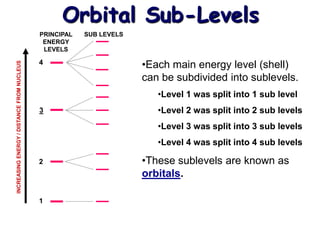
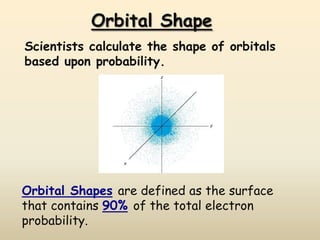
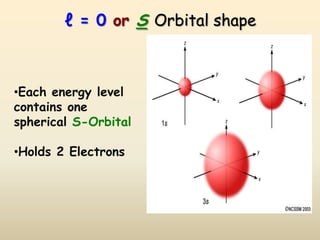
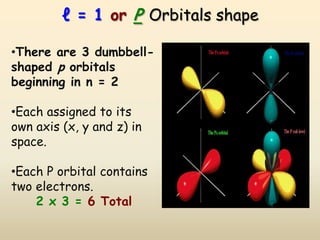
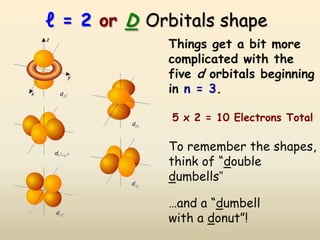
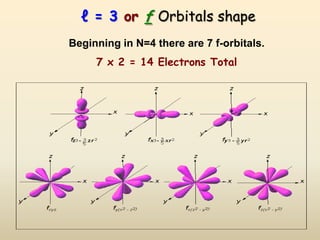
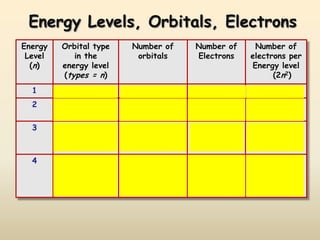
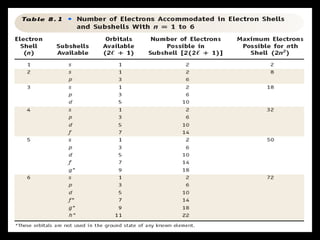
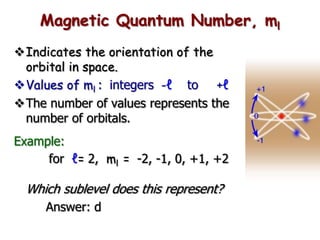
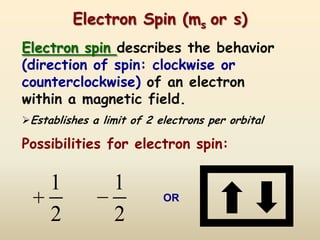
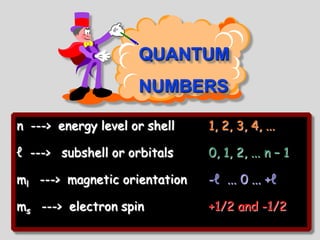
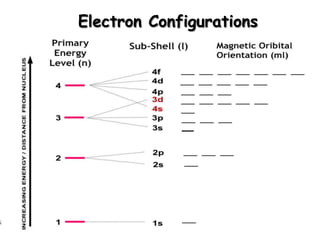
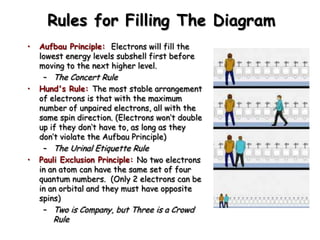
The document summarizes the quantum mechanical model of the atom. It describes how electrons are located in specific energy levels called orbitals, which are regions where electrons are most likely to be found. The location of electrons is determined by four quantum numbers - principal, azimuthal, magnetic, and spin. Electrons fill these orbitals based on specific rules, such as the Aufbau principle and Hund's rule, resulting in unique electron configurations for each element.












![Element Configuration Orbital notation Noble gas
notation notation
Lithium 1s22s1 [He]2s1
____ ____ ____ ____ ____
1s 2s 2p
Beryllium 1s22s2 [He]2s2
____ ____ ____ ____ ____
1s 2s 2p
Boron 1s22s22p1 [He]2s2p1
____ ____ ____ ____ ____
1s 2s 2p
Carbon 1s22s22p2 [He]2s2p2
____ ____ ____ ____ ____
1s 2s 2p
Nitrogen 1s22s22p3 [He]2s2p3
____ ____ ____ ____ ____
1s 2s 2p
Oxygen 1s22s22p4 [He]2s2p4
____ ____ ____ ____ ____
1s 2s 2p
Fluorine 1s22s22p5 [He]2s2p5
____ ____ ____ ____ ____
1s 2s 2p
Neon 1s22s22p6 [He]2s2p6
____ ____ ____ ____ ____
1s 2s 2p](https://image.slidesharecdn.com/quantummoleculartheory-130214052403-phpapp01/85/QMM-38-320.jpg)