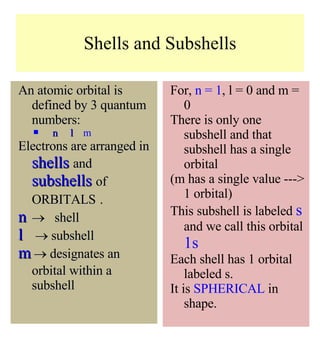
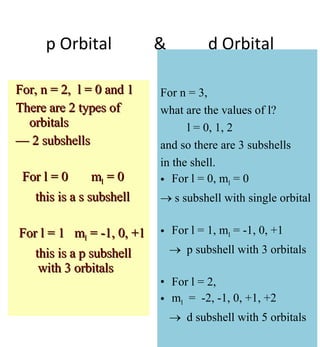
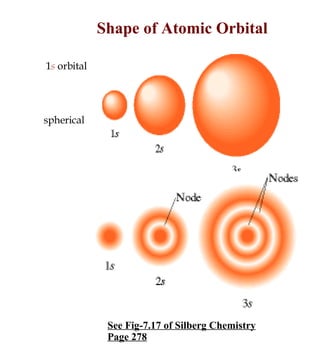
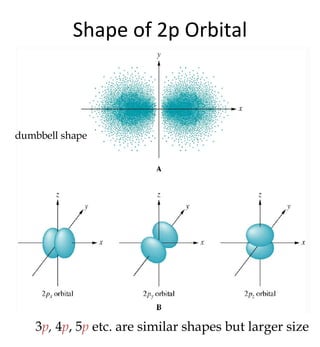
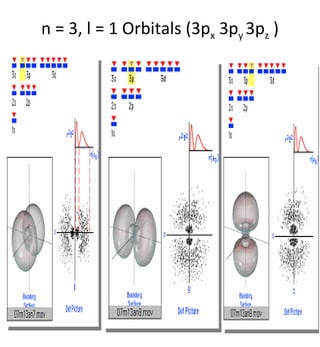
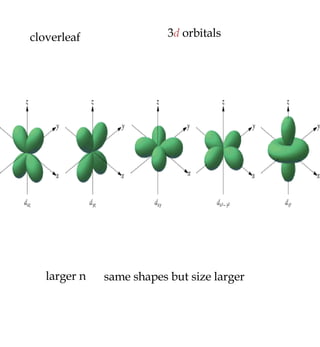
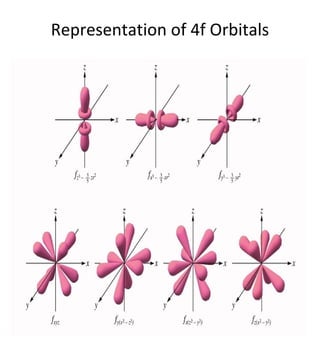
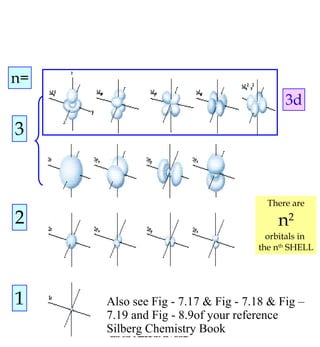
1) The document discusses the electronic configuration of atoms, including the development of wave mechanics and quantum theory to explain the structure of atoms. It introduces concepts like the de Broglie wavelength, quantum numbers, atomic orbitals and shapes, Pauli's exclusion principle, and Hund's rule for electron configuration.
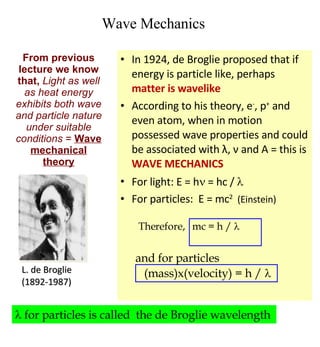
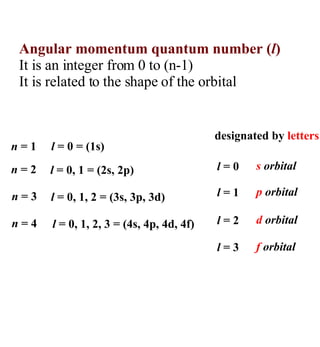
2) Key scientists discussed include de Broglie, Heisenberg, Schrodinger, Pauli, and their contributions to developing models of the atom and allowing prediction of electron configurations.
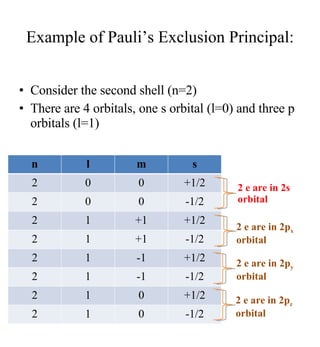
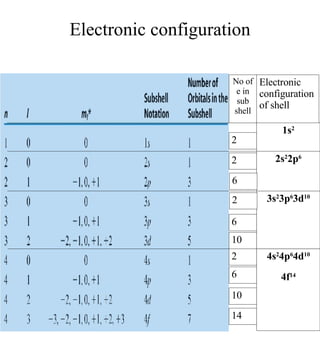
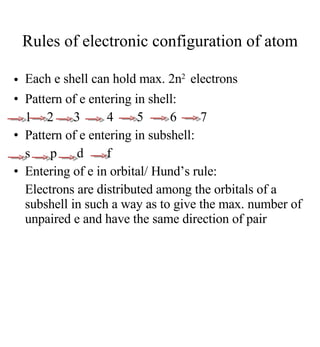
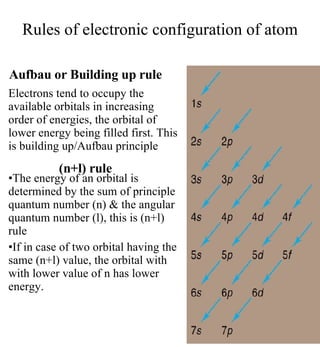
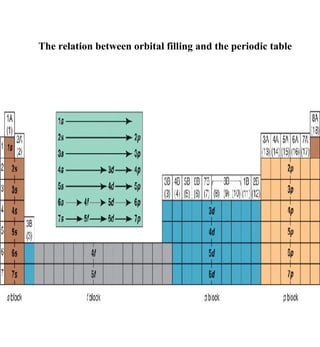
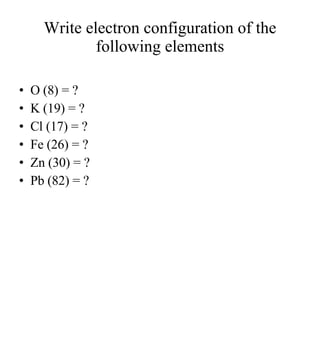
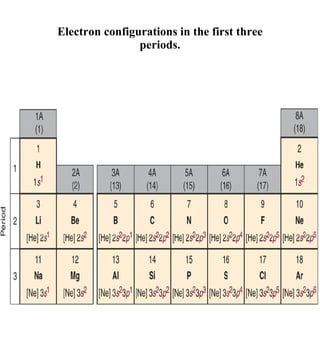
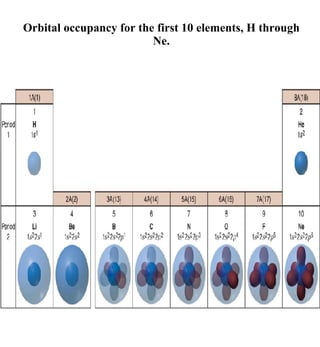
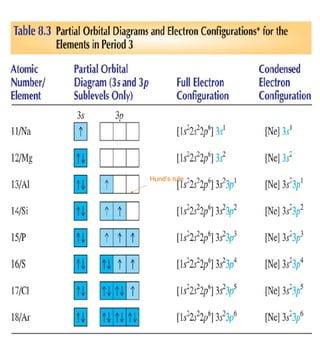
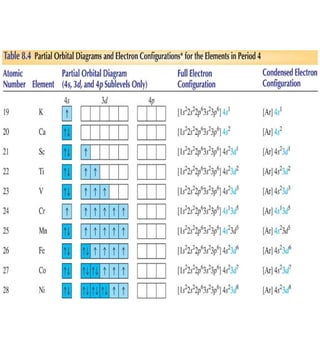
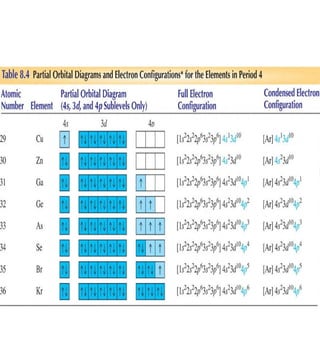
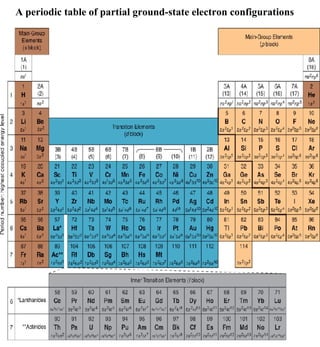
3) The document provides examples of writing out electron configurations for elements and explaining the rules for filling atomic orbitals in the Aufbau principle.