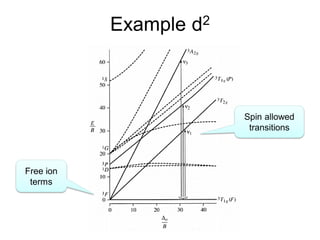
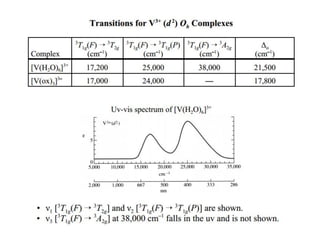
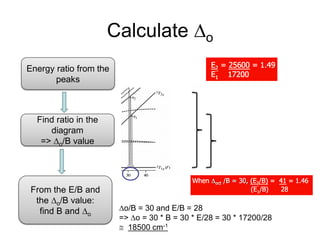
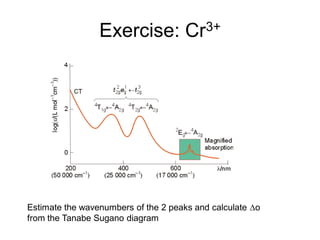
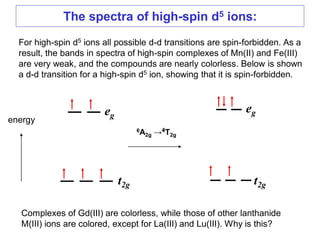
This document discusses UV-VIS spectroscopy of transition metal complexes. It covers:
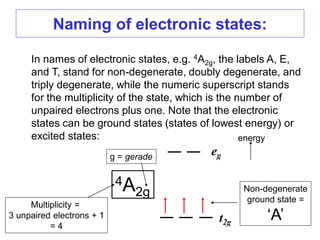
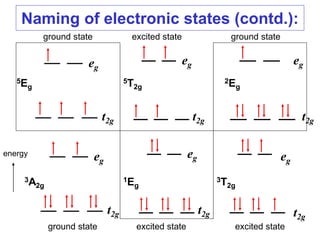
1. The features of electronic spectra that need to be understood, such as naming electronic states and transitions.
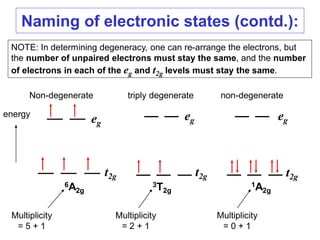
2. The selection rules that govern the intensities of bands in spectra, including the Laporte and spin selection rules. Laporte-allowed and spin-allowed transitions are most intense.
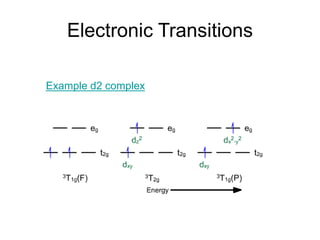
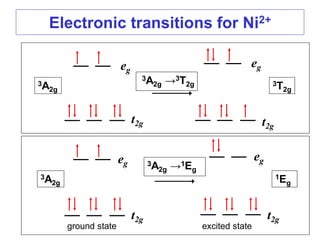
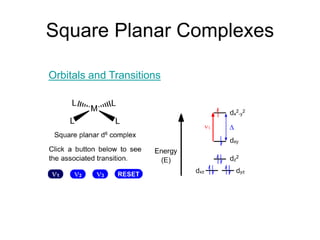
3. Examples of electronic spectra are shown for complexes such as [Ni(H2O)6]2+, and the transitions are explained using both crystal field and molecular orbital theories.
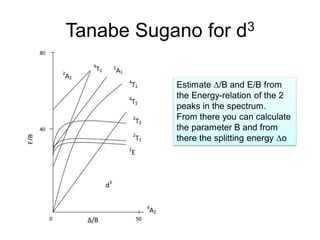
Tanabe-Sugano diagrams are also introduced as a way to determine crystal field splitting parameters from experimental transition energies.

![d-d spectra and MO theory:
3A2g →3T2g
3A2g →1Eg
υ, cm-1
UV
[Ni(NH3)6]2+
visible infrared](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-3-320.jpg)

![visible infraredUV
green
3A2g →3T2g
3A2g →1Eg
[Ni(H2O)6]2+
The electronic spectrum of [Ni(H2O)6]2+:
λ,
The complex looks green, because it absorbs only weakly at 500 nm,
the wavelength of green light.](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-10-320.jpg)
![On the previous slide we saw the two bands due to the
3A2g →3T2g and 3A2g →1Eg transitions. The band at λ =
1180 nm which is the 3A2g →3T2g transition shown below,
corresponds to Δ for the complex. This is usually
expressed as Δ in cm-1 = (1/λ(nm)) x 107 = 8500 cm-1.
The electronic spectrum of [Ni(H2O)6]2+:
eg
eg
t2g t2g
3A2g →3T2g3A2g
3T2gΔ
= Δ
= 8500
cm-1](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-11-320.jpg)
![Note the weak band at 620 nm that corresponds to the
3A2g →1Eg transition. The electron that is excited moves
within the eg level, so that the energy does not involve Δ,
but depends on the value of P, the spin-pairing energy.
The point of interest is why this band is so weak, as
discussed on the next slide.
The electronic spectrum of [Ni(H2O)6]2+:
eg
eg
t2g t2g
3A2g →1Eg3A2g
1EgΔ
= 16100
cm-1](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-12-320.jpg)
![The electronic spectrum of [Ni(H2O)6]2+:
The two peaks at higher energy resemble the 3A2g→3T2g transition, but
involve differences in magnetic quantum numbers of the d-orbitals,
and are labeled as 3A2g→3T1g(F) and 3A2g→3T1g(P) to reflect this:
3A2g →3T2g
3A2g →3T1g(F)
3A2g →3T1g(P)
3A2g →1Eg
λ,
[Ni(H2O)6]2+](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-13-320.jpg)
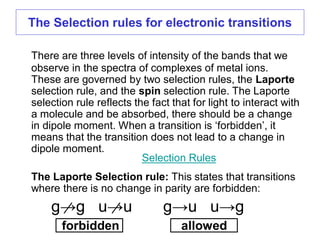
![The Selection rules for electronic transitions
3A2g →3T2g
Charge-transfer band – Laporte and spin allowed – very intense
[Ni(H2O)6]2+
a
b c
3A2g →1Eg Laporte and spin forbidden – very weak
a, b, and c, Laporte
forbidden, spin
allowed, inter-
mediate intensity](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-16-320.jpg)
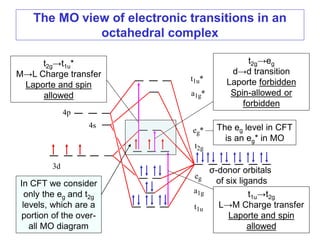
![The three types of bands present in e.g. [Ni(H2O)6]2+ are:
1) Laporte-allowed plus spin allowed charge transfer
bands of very high intensity
2) Laporte-forbidden plus spin-allowed d→d transitions
(e.g. 3A2g→3T2g) of moderate intensity
3) Laporte forbidden plus spin-forbidden d→d transitions
(3A2g→1Eg) of very low intensity.
The Intensity of bands in complexes of d-block ions:](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-17-320.jpg)

![Mixing of states: Comparison of [Ni(H2O)6]2+ and [Ni(en)3]2+:
[Ni(H2O)6]2+
[Ni(en)3]2+
3A2g →3T2g
3A2g →3T2g(F)
The spin-forbidden 3A2g →1Eg is close to the spin-allowed
3A2g →3T2g(F) and ‘borrows’ intensity by mixing of states
The spin-forbidden 3A2g →1Eg is not close
to any spin allowed band and is very weak
3A2g →1Eg
Note: The two spectra are
drawn on the same graph
for ease of comparison.](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-21-320.jpg)
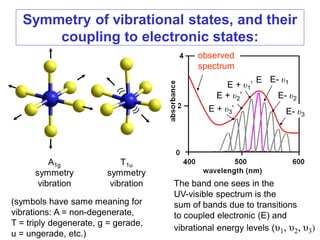
![The spectra of complexes of tetrahedral
metal ions:
A tetrahedron has no center of symmetry, and so orbitals in
such symmetry cannot be gerade. Hence the d-levels in a
tetrahedral complex are e and t2, with no ‘g’ for gerade.
This largely overcomes the Laporte selection rules, so that
tetrahedral complexes tend to be very intense in color. Thus,
we see that dissolving CoCl2 in water produces a pale pink
solution of [Co(H2O)6]2+, but in alcohol tetrahedral
[CoCl2(CH3CH2OH)2] forms, which is a very intense blue
color. This remarkable difference in the spectra of
octahedral and tetrahedral complexes is seen on the next
slide:](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-26-320.jpg)
![The spectra of octahedral [Co(H2O)6]2+ and
tetrahedral [CoCl4]2- ions:
[CoCl4]2-
[Co(H2O)6]2+
The spectra at left
show the very intense
d-d bands in the blue
tetrahedral complex
[CoCl4]2-, as compared
with the much weaker
band in the pink
octahedral complex
[Co(H2O)6]2+. This
difference arises
because the Td com-
plex has no center of
symmetry, helping to
overcome the g→g
Laporte selection rule.](https://image.slidesharecdn.com/spectroscopicmethodsuv-vistmcomplexes-170821103701/85/Spectroscopic-methods-uv-vis-transition-metal-complexes-27-320.jpg)