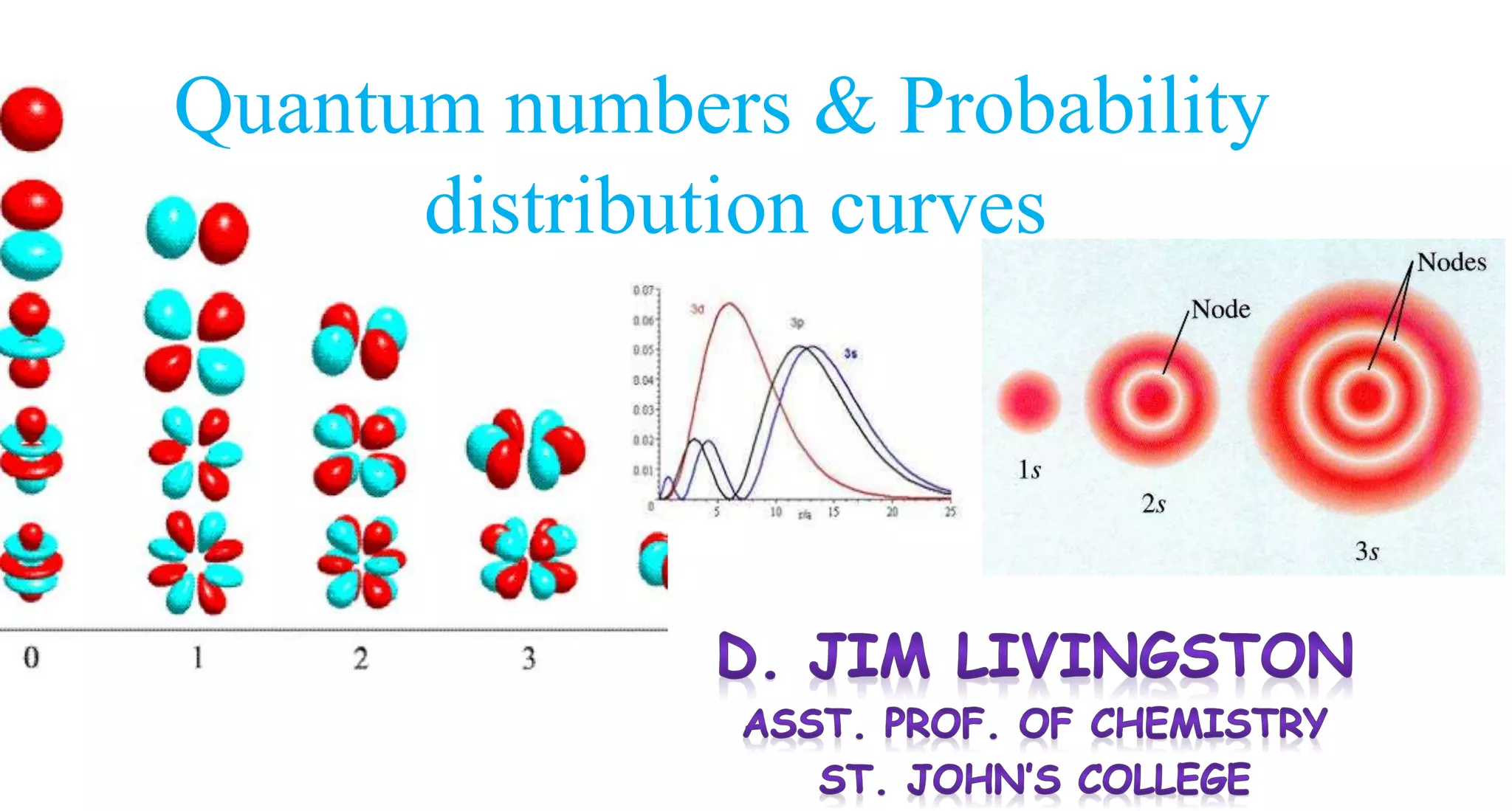
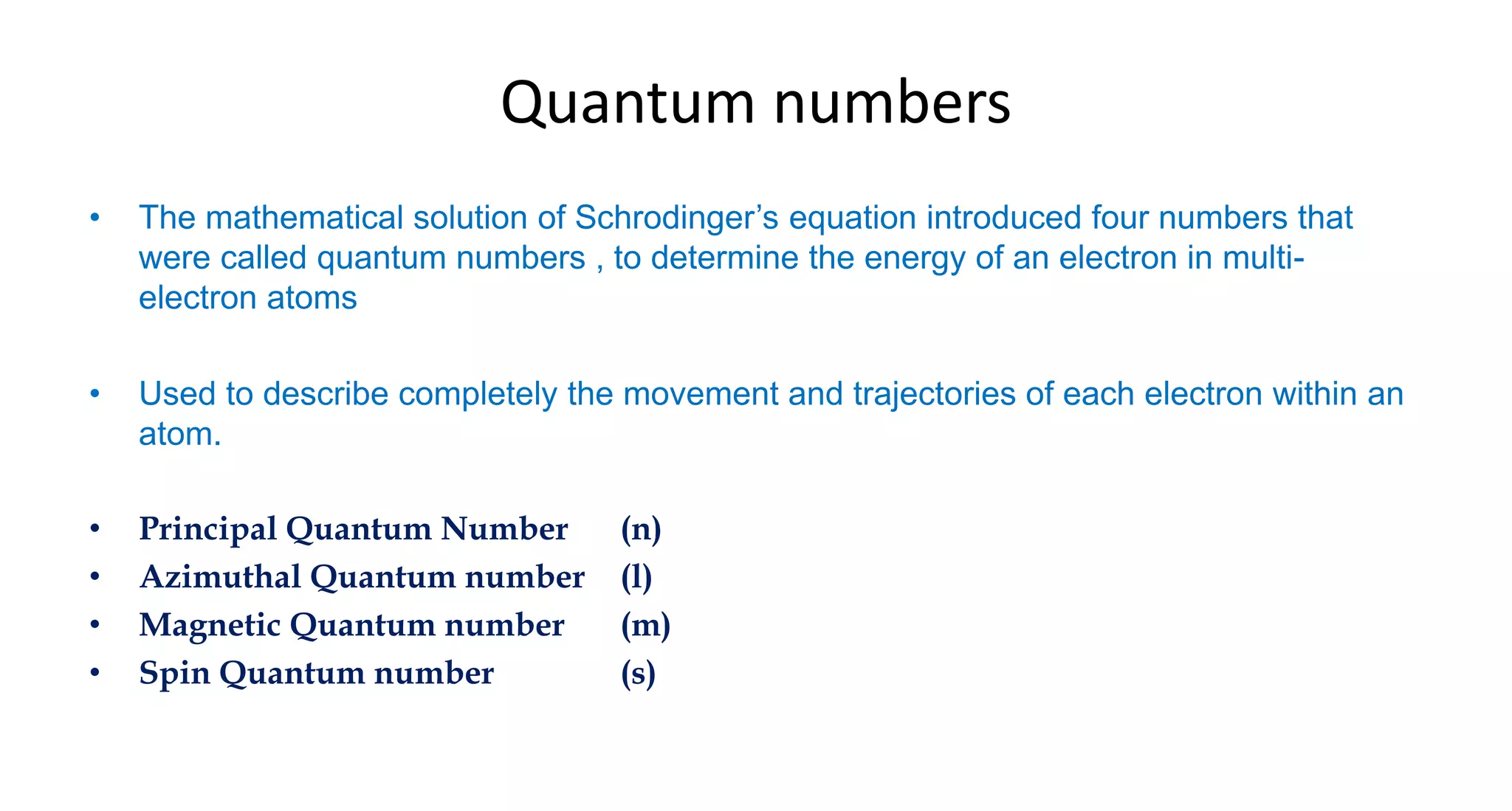
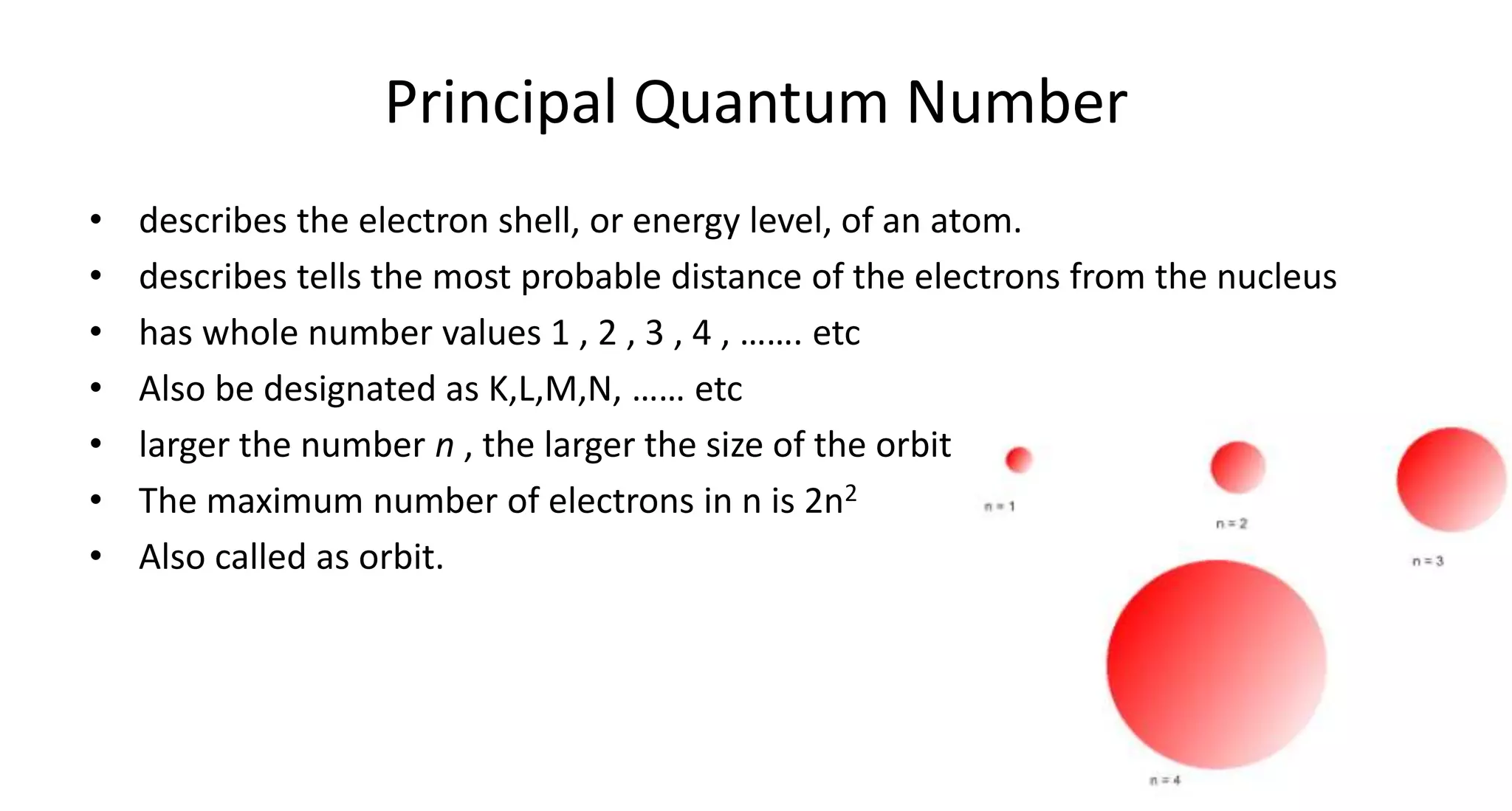
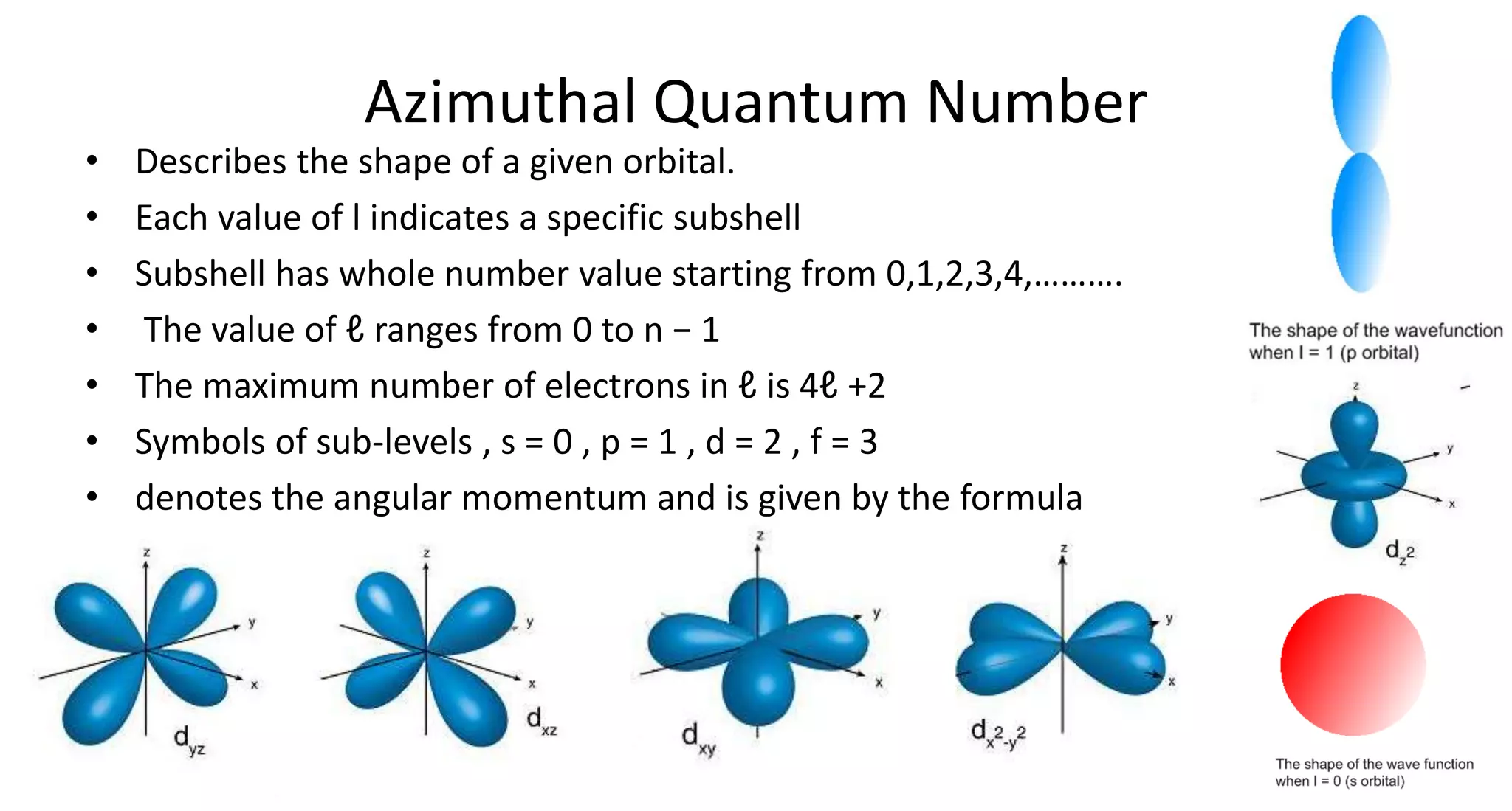
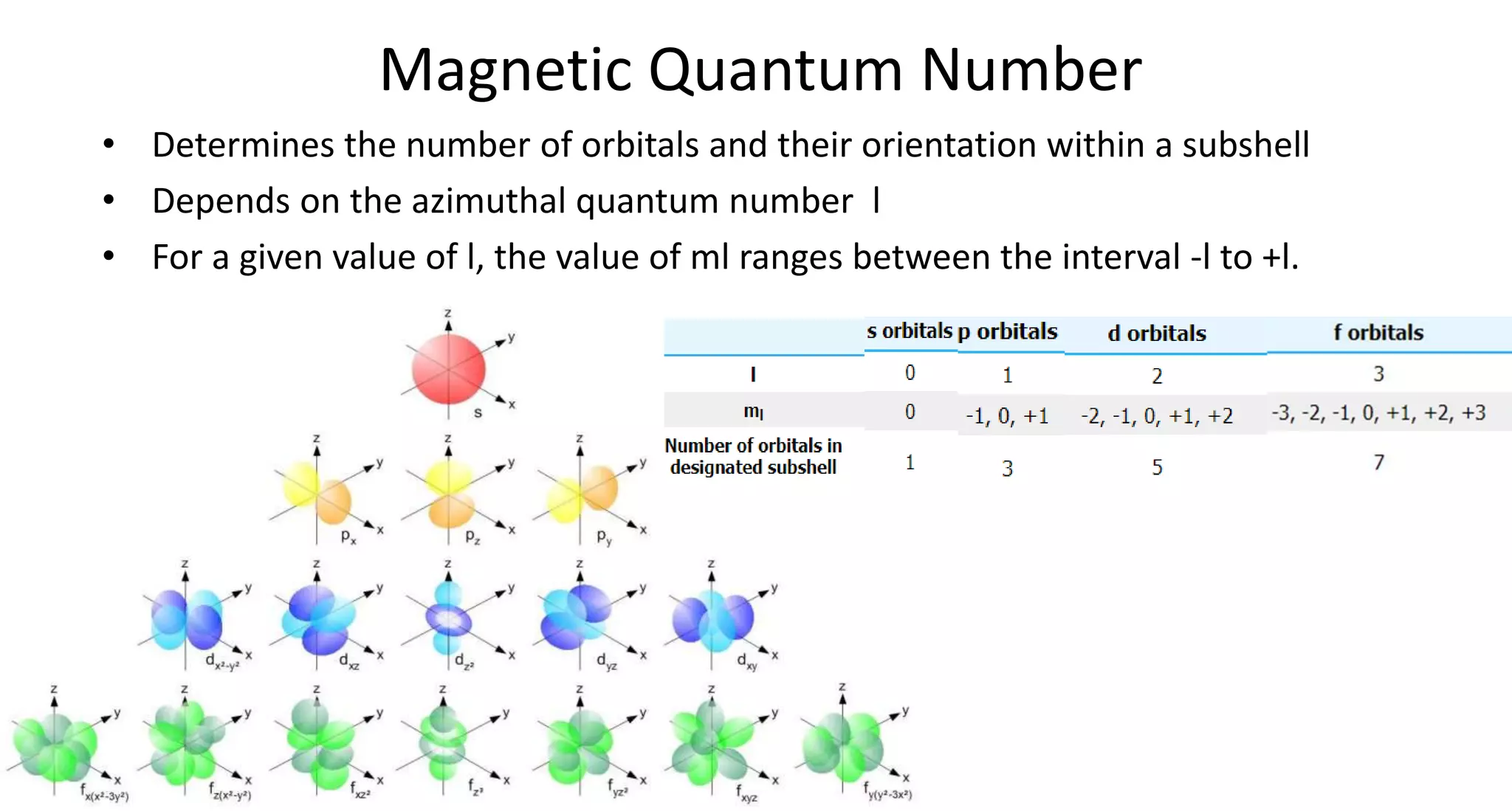
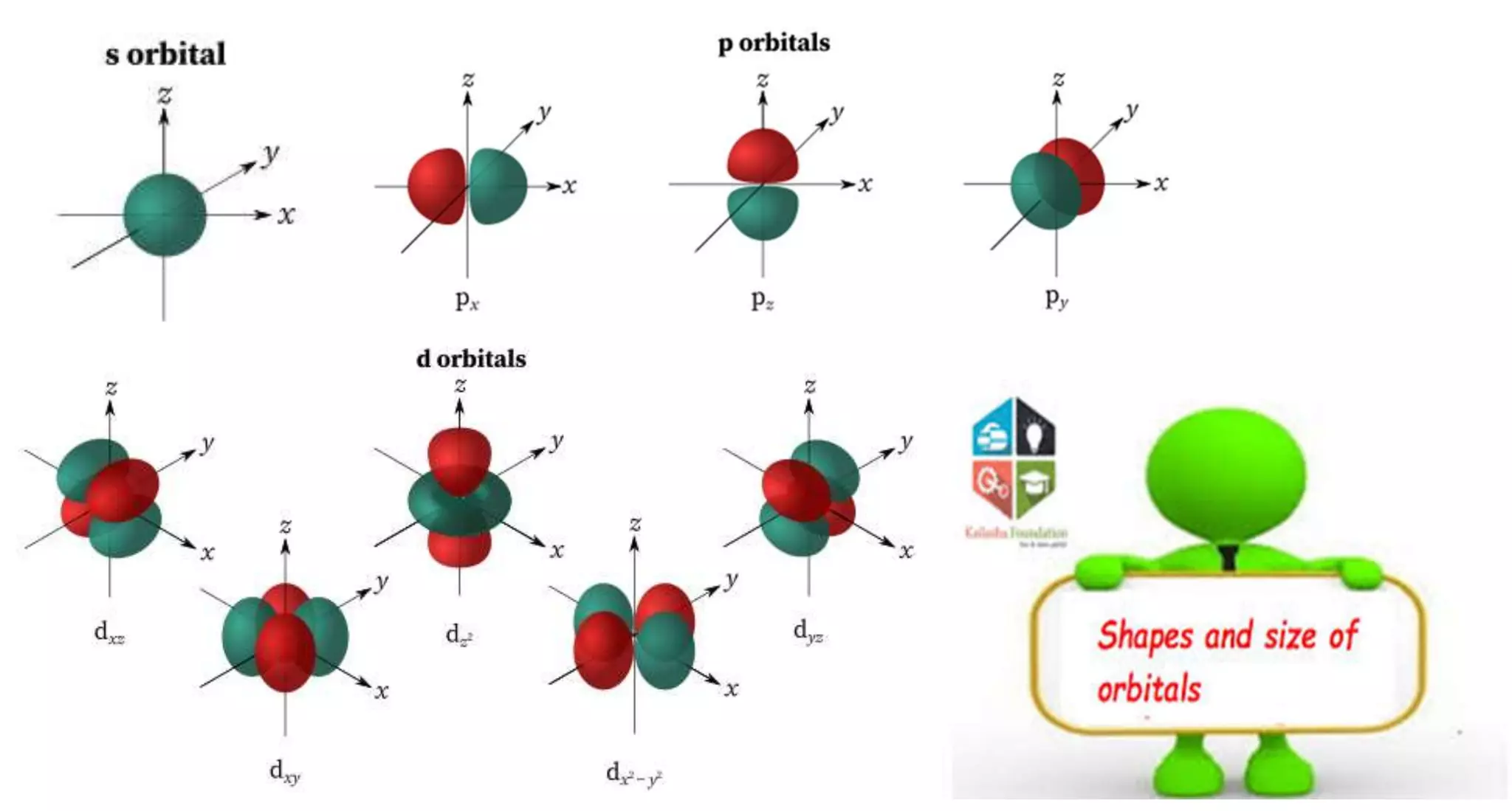
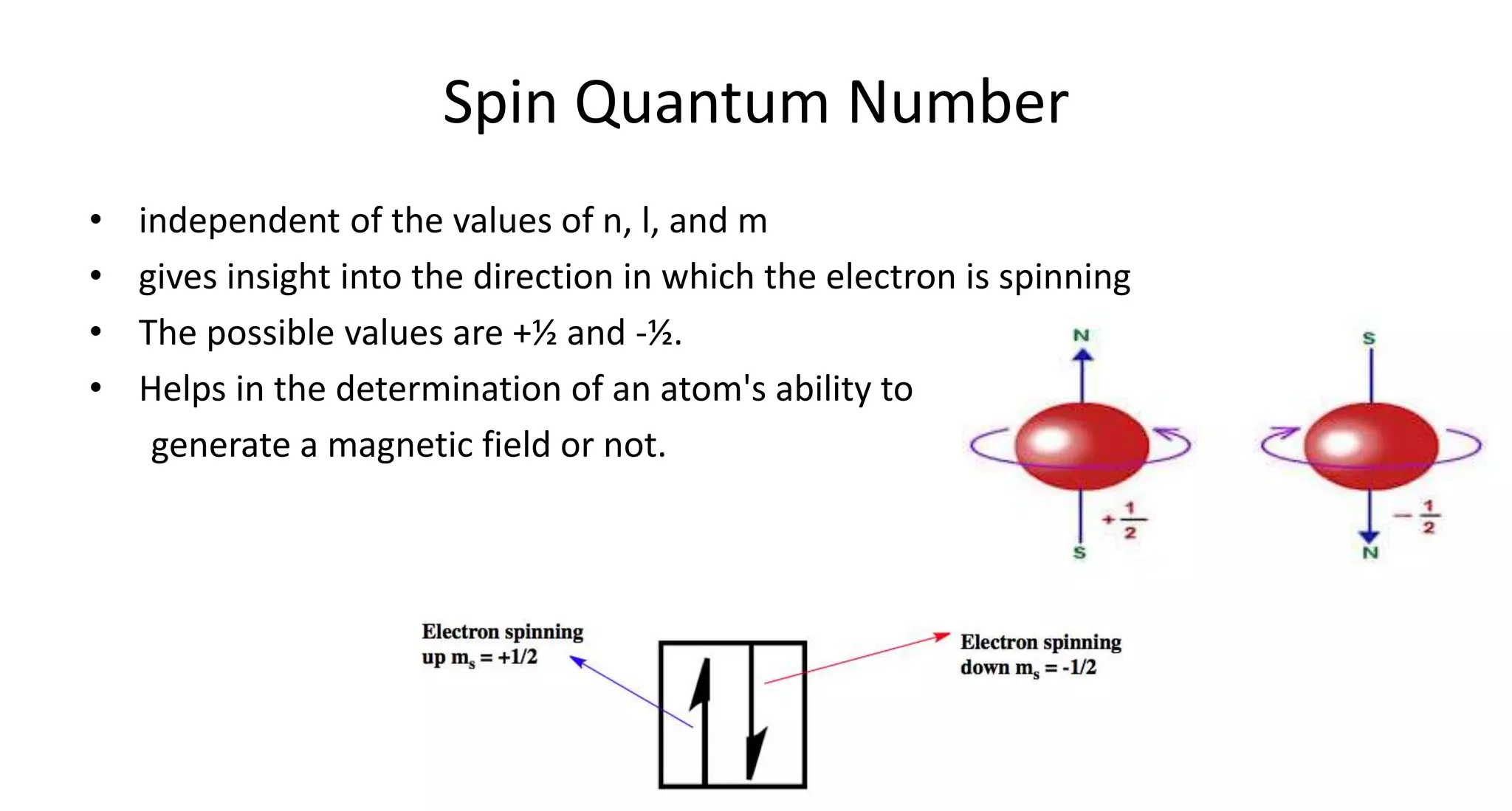
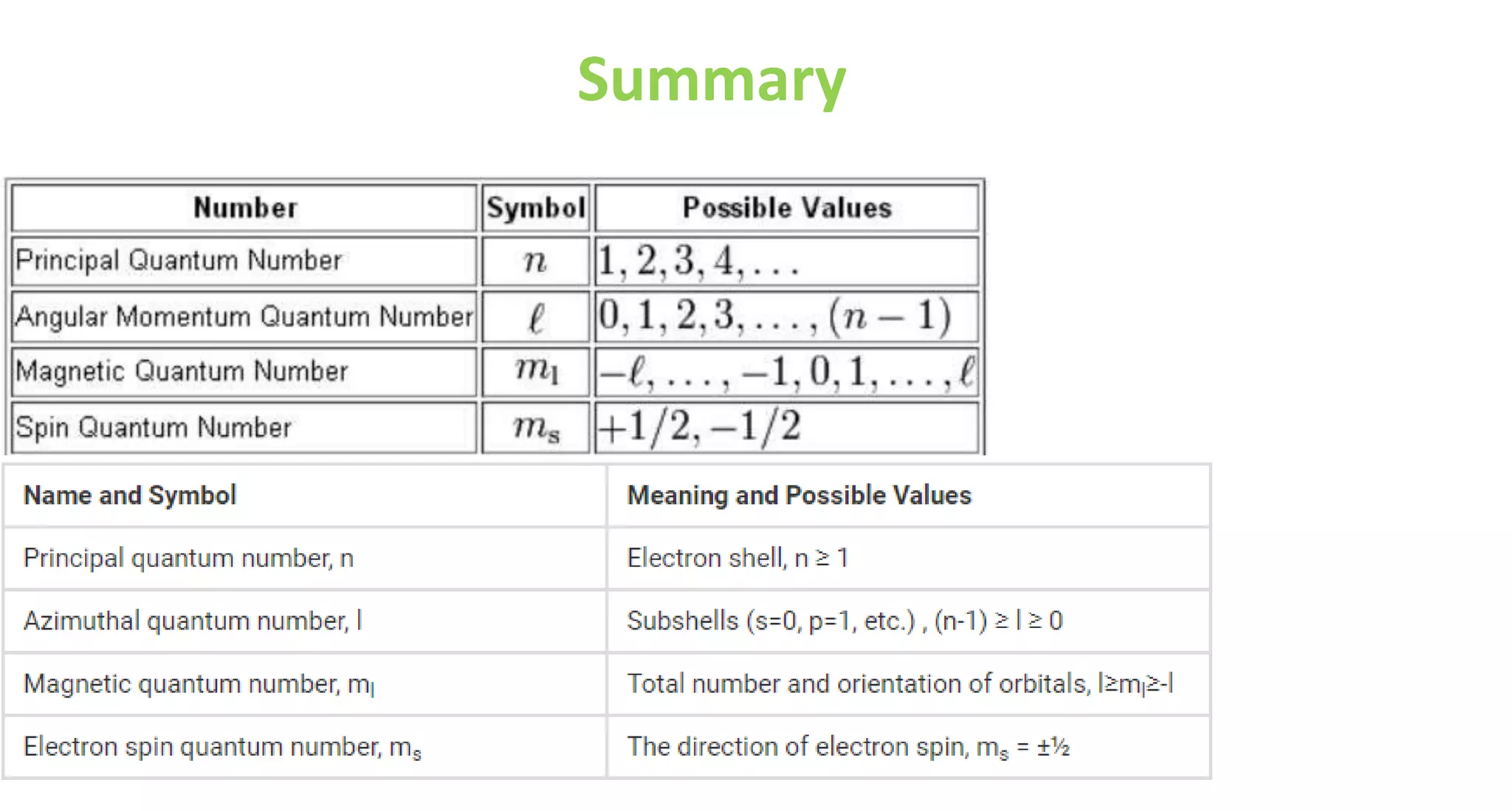
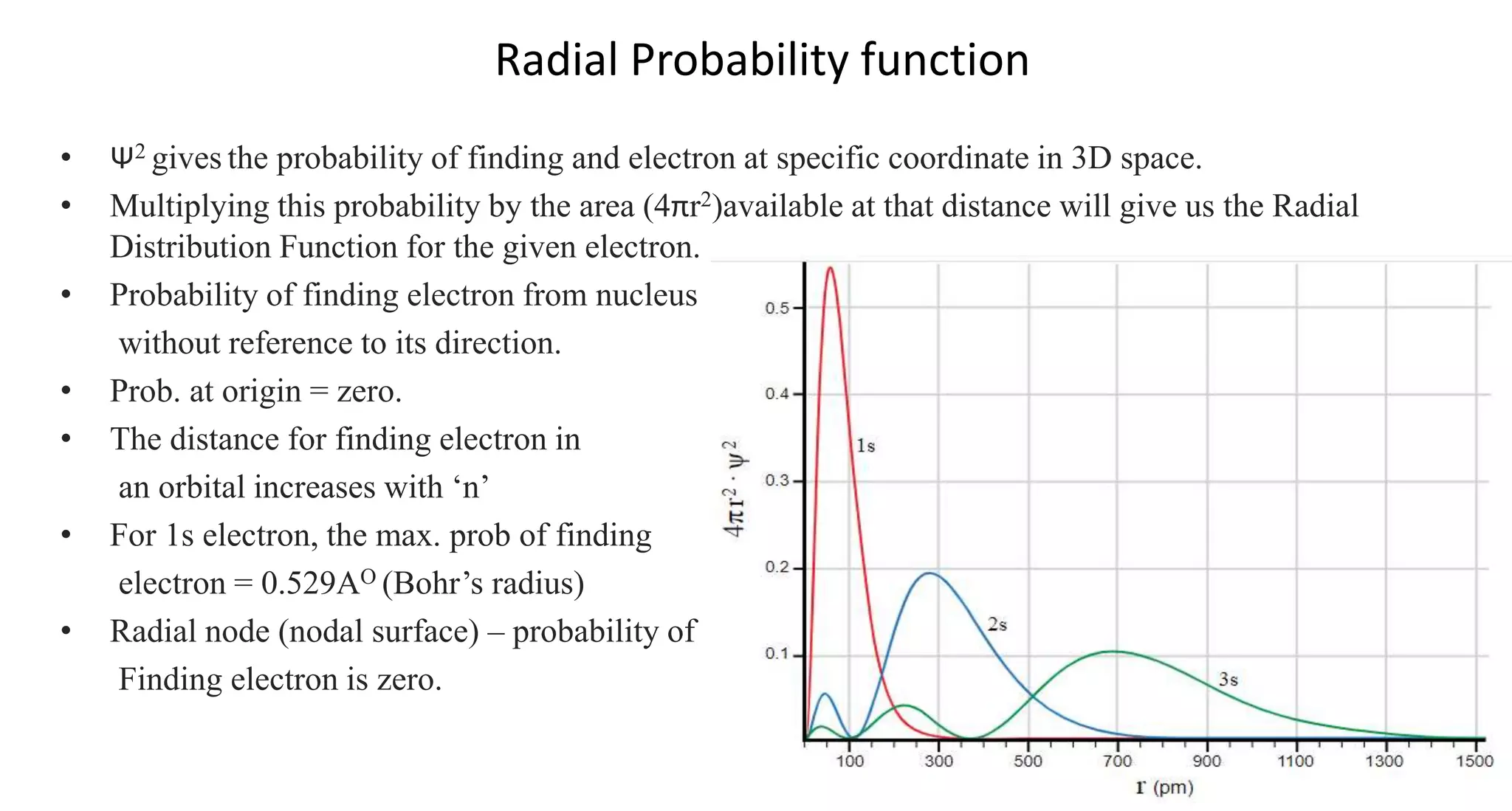
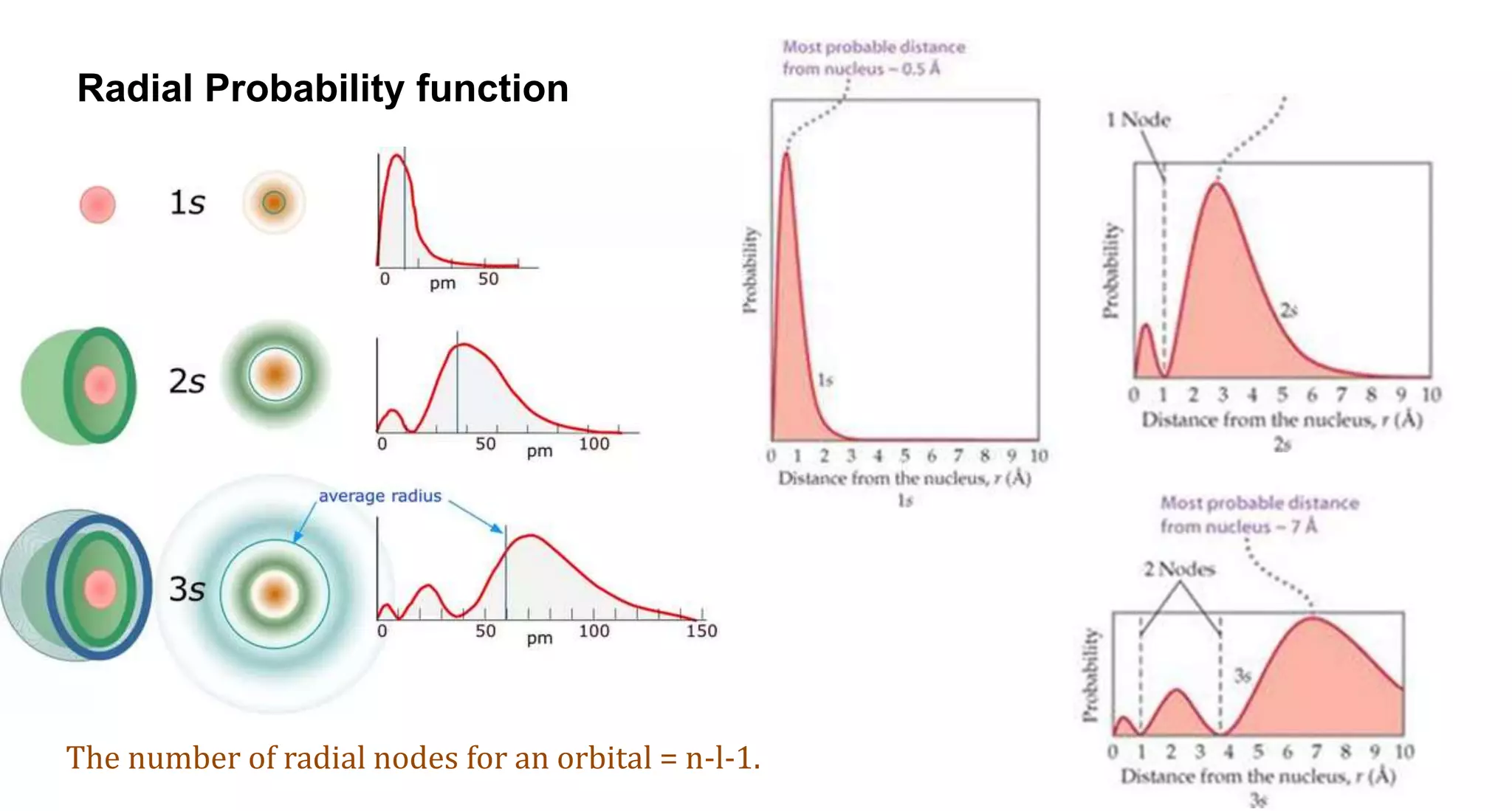
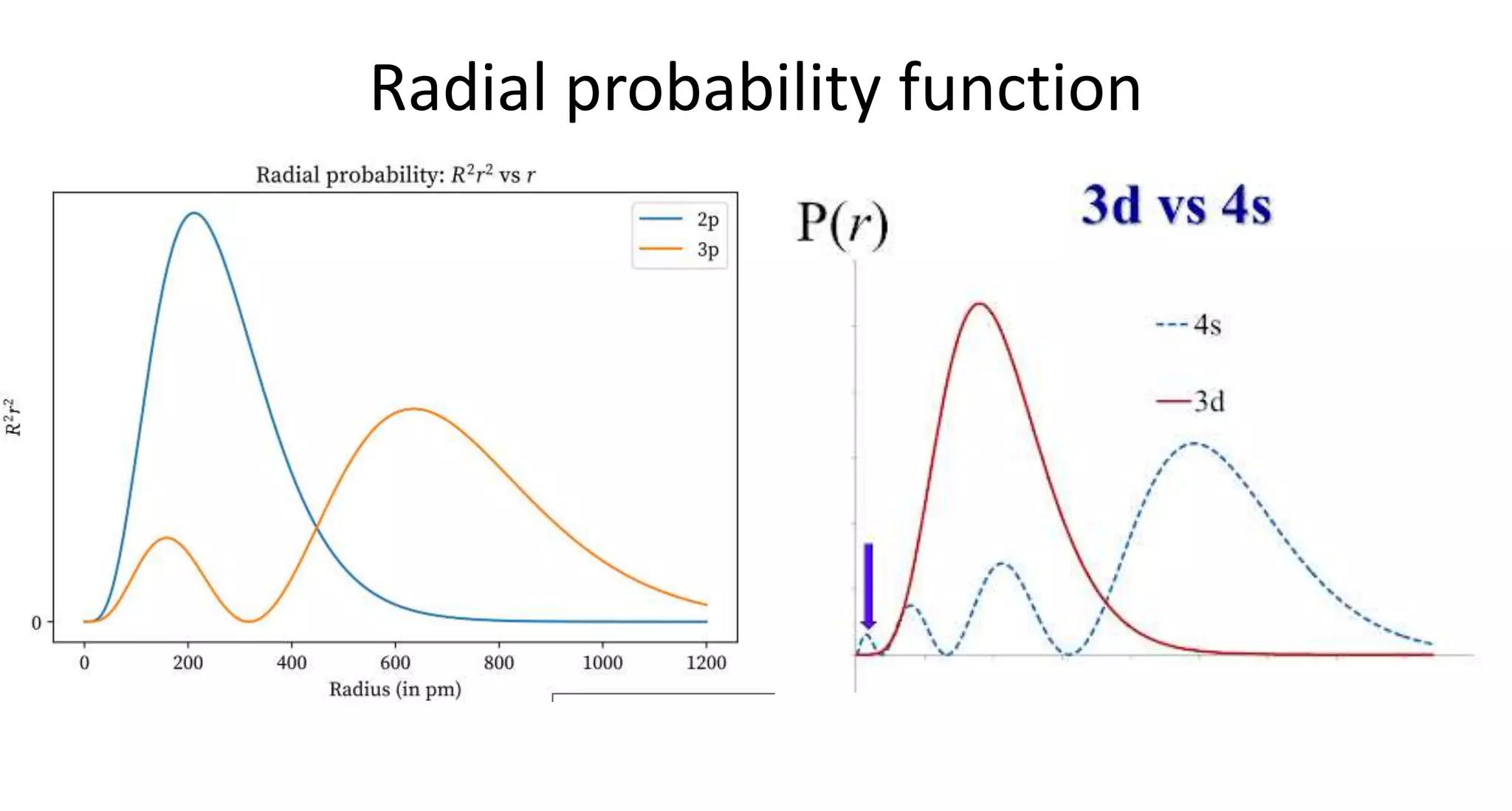
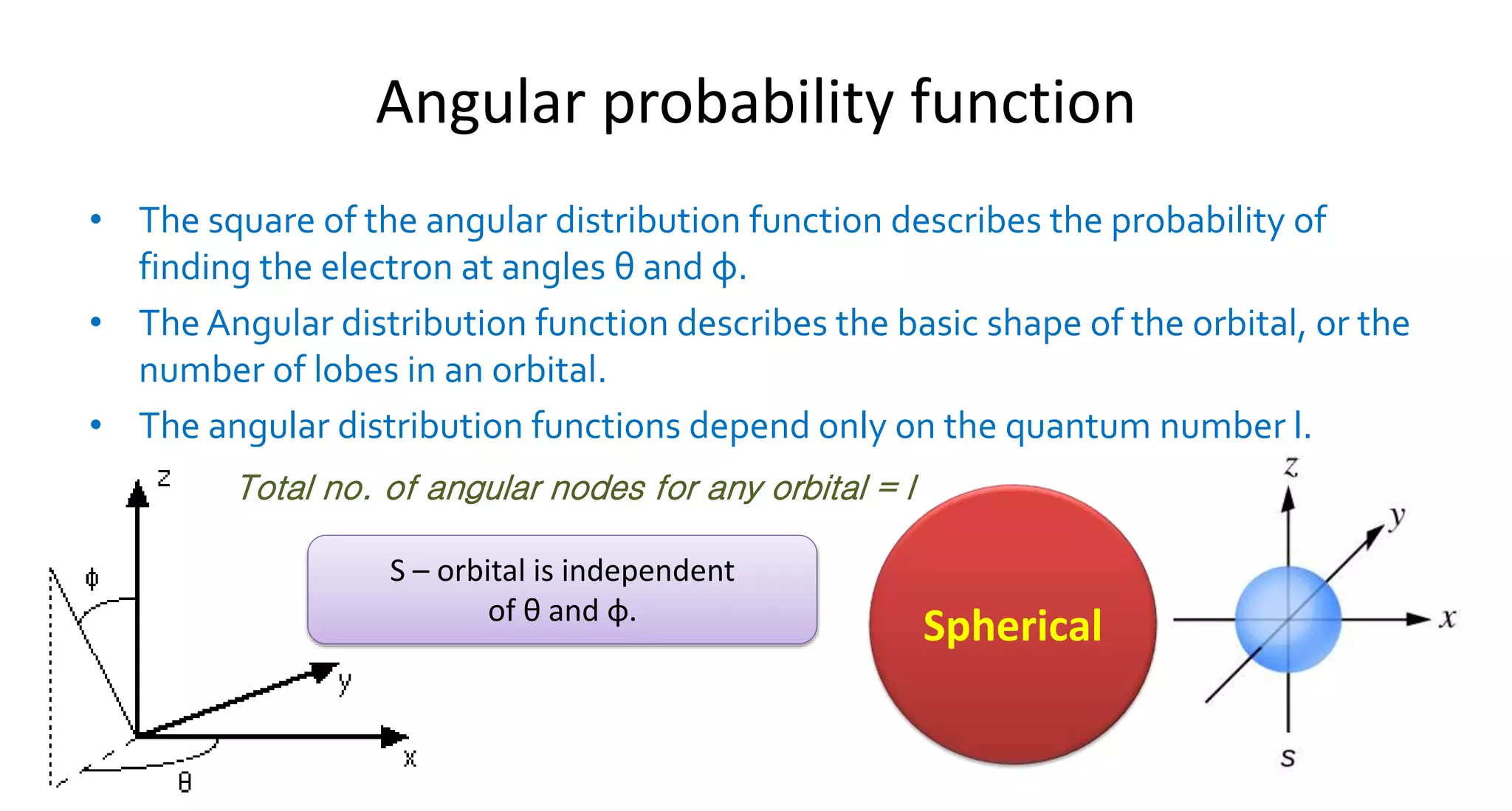
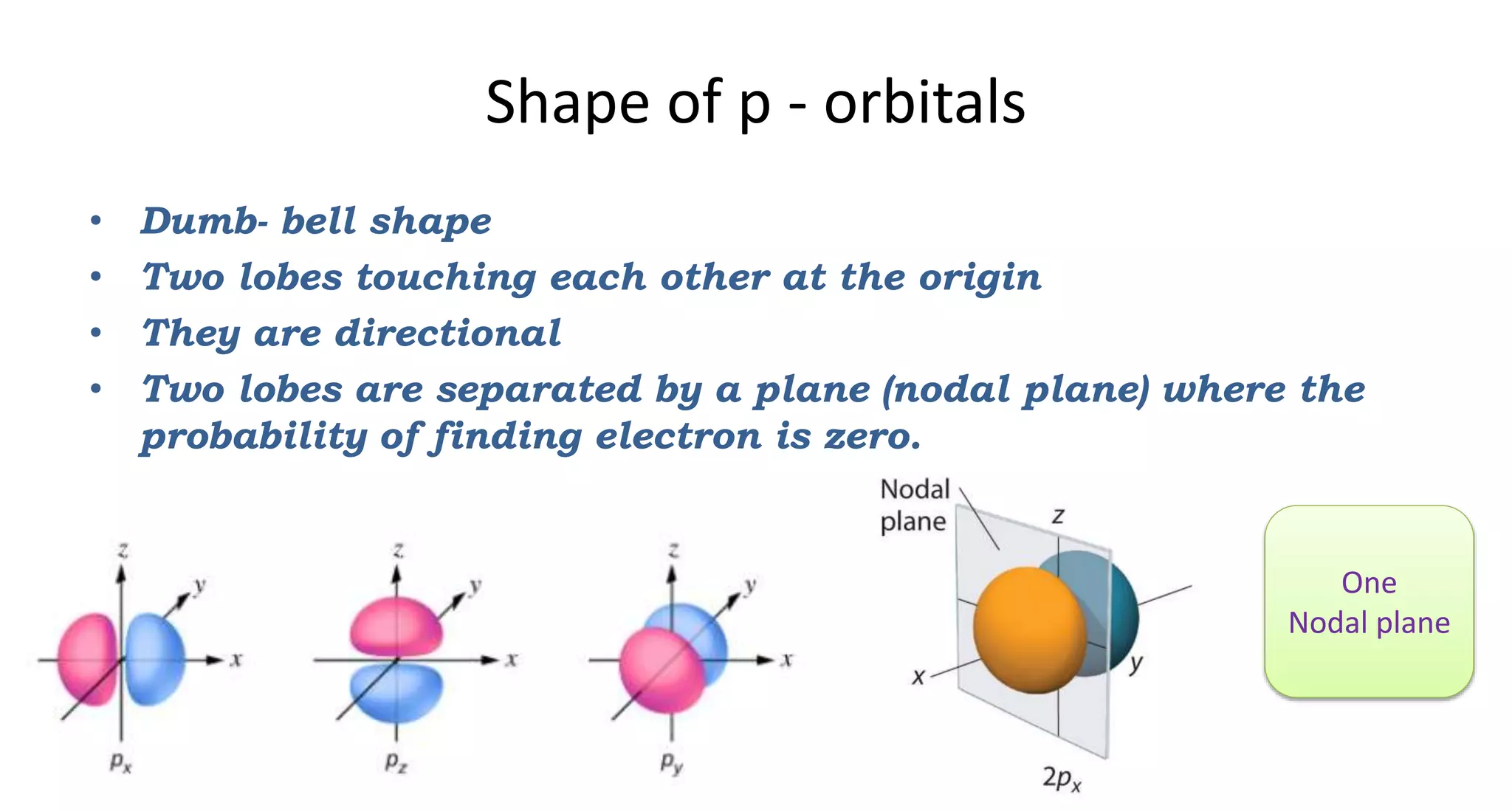
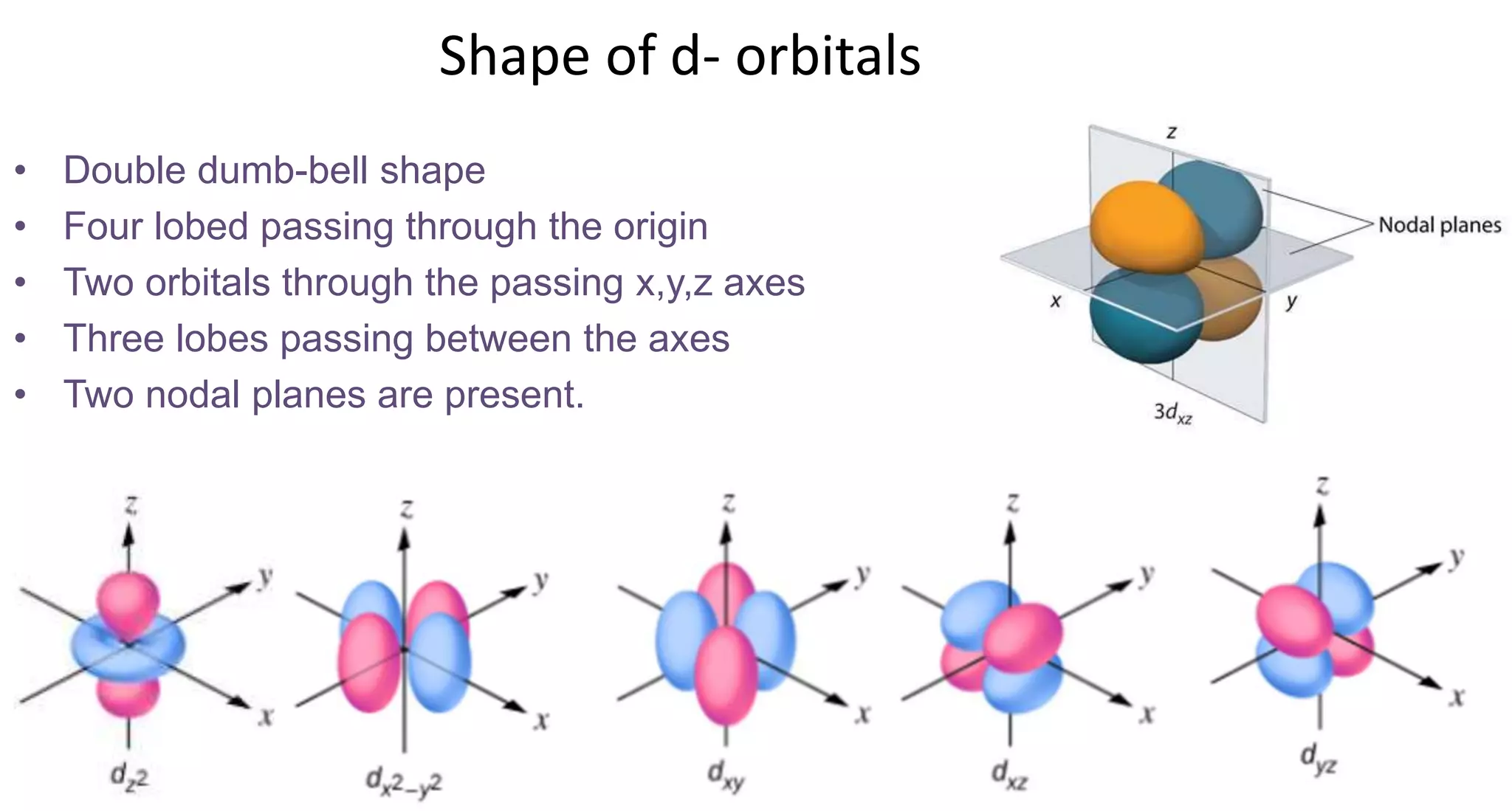
This document discusses quantum numbers and probability distribution curves. It introduces the four quantum numbers - principal, azimuthal, magnetic, and spin - that are used to determine the energy of electrons in atoms. It describes each quantum number and what properties they indicate, such as the electron shell or subshell, orbital shape, and electron spin. It then discusses radial and angular probability functions, which describe the probability of finding an electron at different distances from the nucleus or at different angles, helping to determine the shape and number of lobes in an orbital.