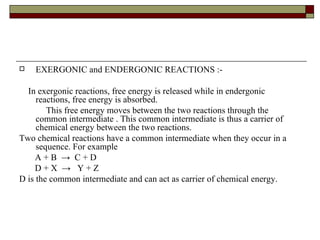
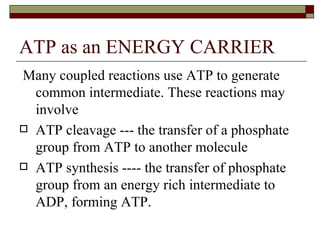
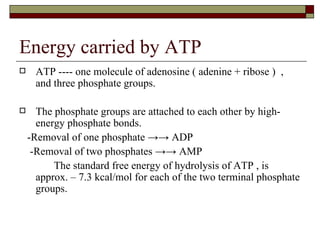
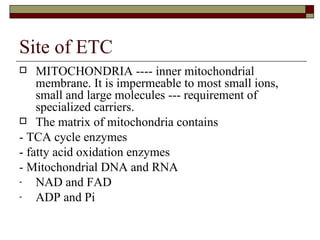
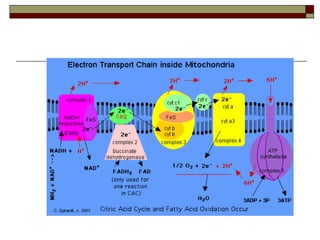
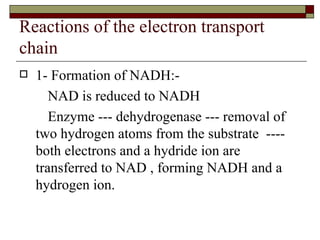
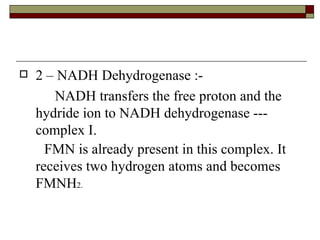
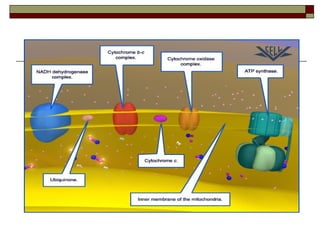
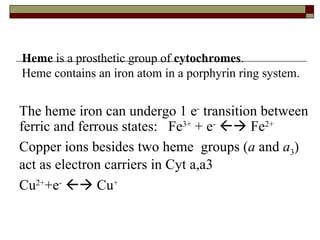
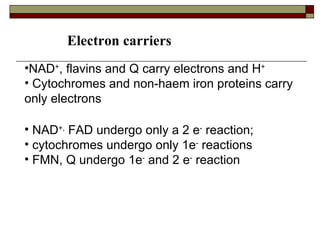
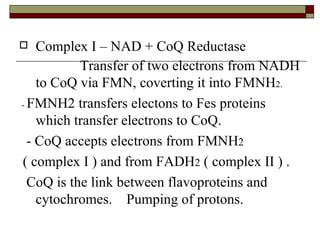
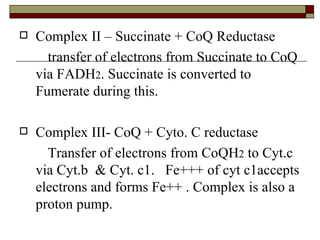
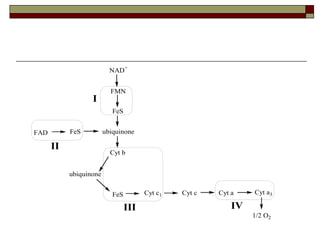
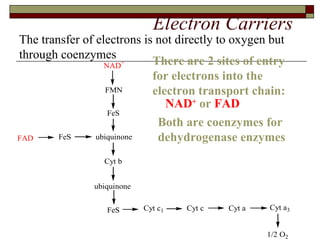
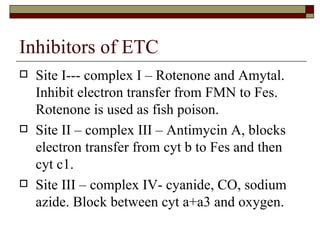
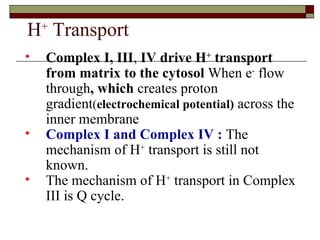
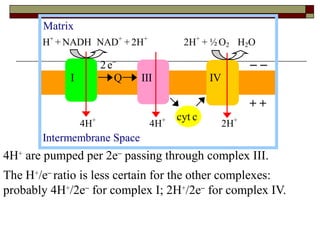
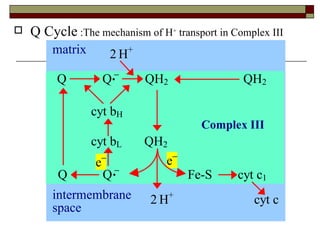
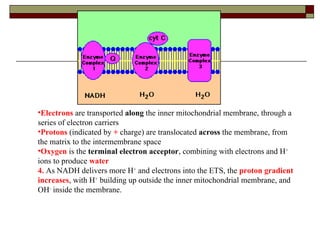
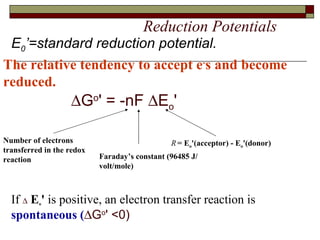
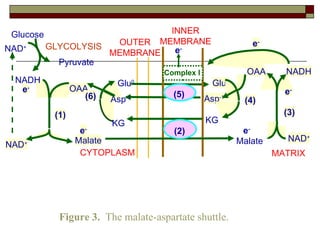
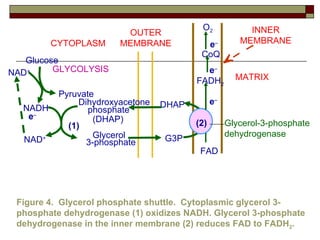
Biological oxidation involves the loss of electrons and/or hydrogen atoms from a molecule through enzymatic reactions. There are three classes of biological oxidation: loss of electrons, loss of hydrogen atoms, or addition of oxygen atoms. During electron transport chain reactions, electrons from energy-rich molecules are transferred through electron carriers like NADH and FADH2 to oxygen. This releases free energy used to generate a proton gradient across the inner mitochondrial membrane and to synthesize ATP through oxidative phosphorylation. ATP acts as an energy currency by transferring phosphate groups from energy-rich intermediates to ADP.