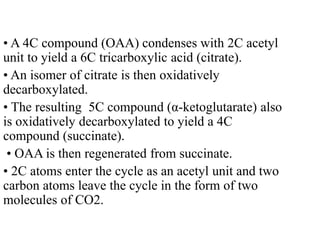
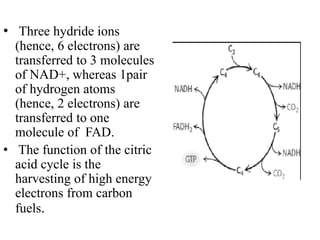
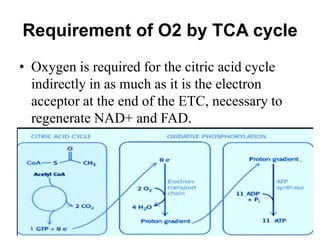
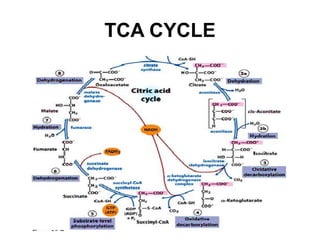
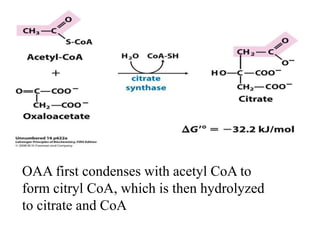
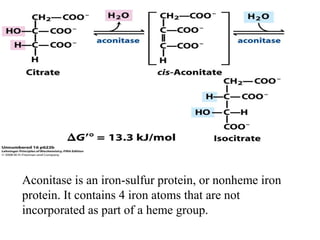
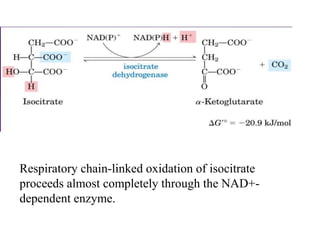
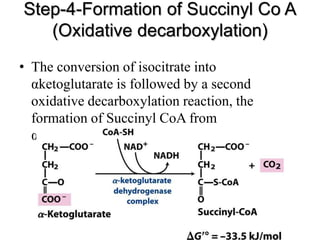
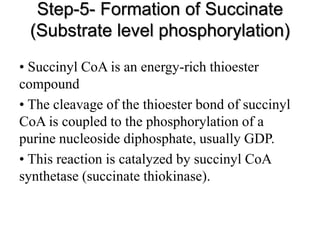
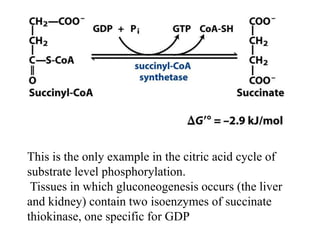
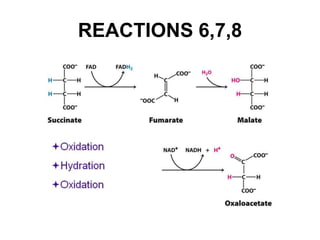
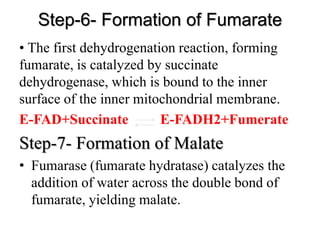
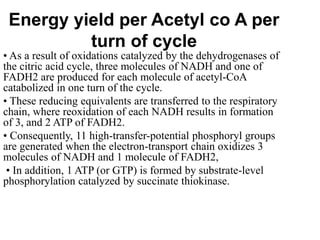
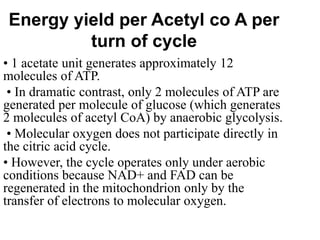
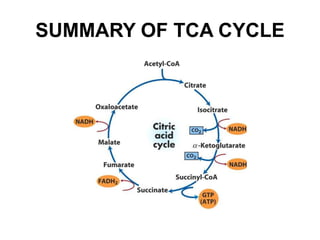
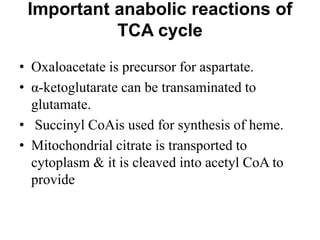
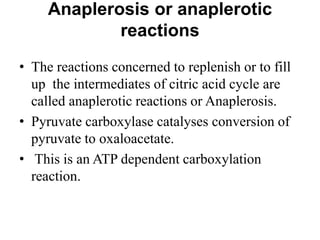
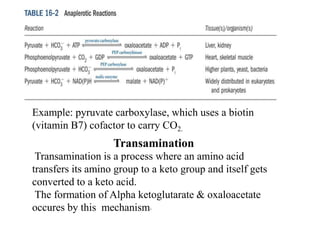
The citric acid cycle (TCA cycle) is a central metabolic pathway that oxidizes acetyl-CoA derived from carbohydrates, fats, and proteins, producing carbon dioxide and reducing equivalents in the form of NADH and FADH2. The TCA cycle consists of 8 steps that occur in the mitochondrial matrix and ultimately generate 12 ATP per acetyl-CoA molecule. Regulation of the TCA cycle occurs through three enzymes and is influenced by levels of ATP, NADH, and other metabolites. While the TCA cycle functions primarily in energy production, it also interfaces with many anabolic pathways through various intermediates.