Embed presentation
Downloaded 22 times

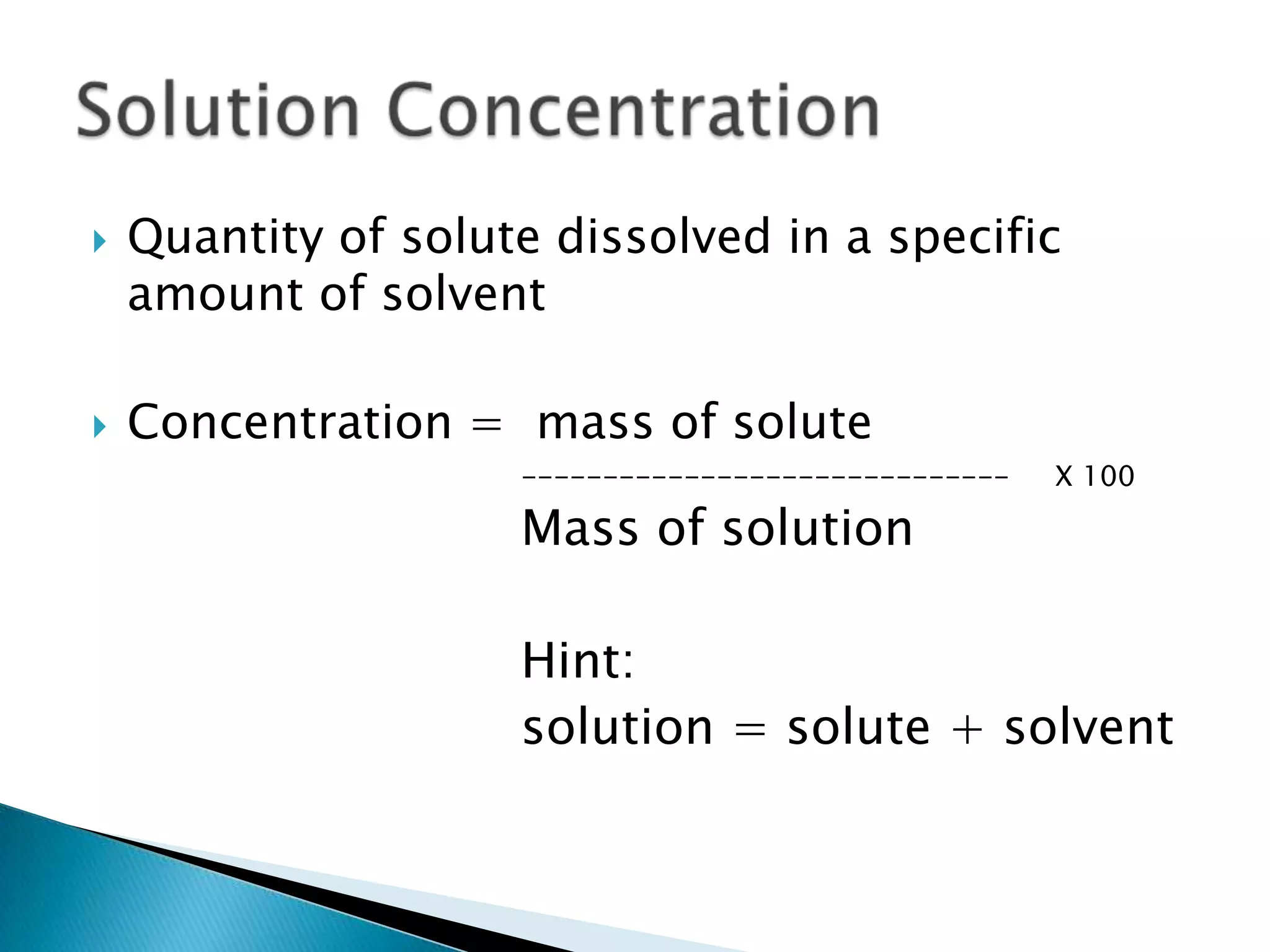


This document discusses different units for expressing concentration of solutions, including percent, parts per hundred (pph), and parts per million (ppm). It provides an example of calculating the concentration in percent by mass of a solution containing 25 g of sucrose dissolved in 175 g of water.



