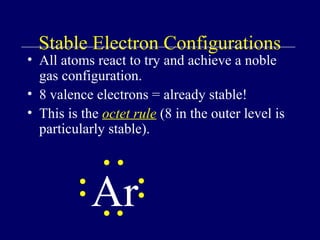
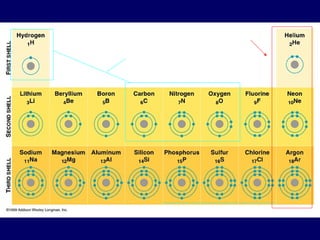
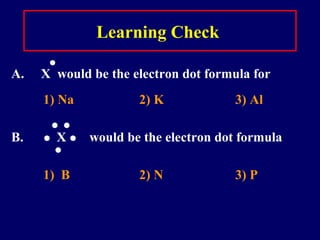
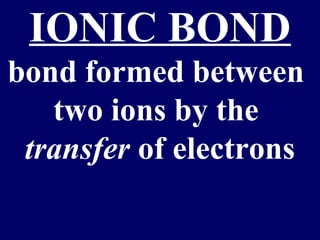
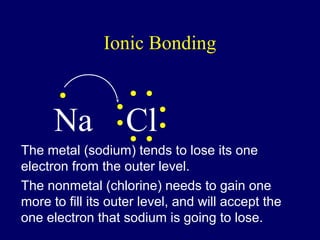
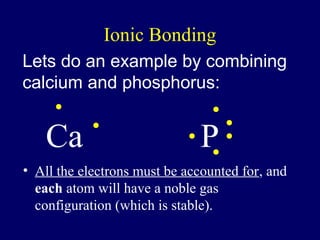
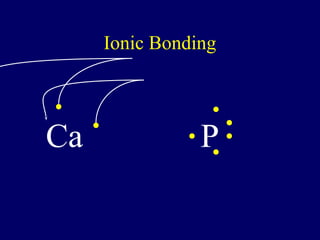
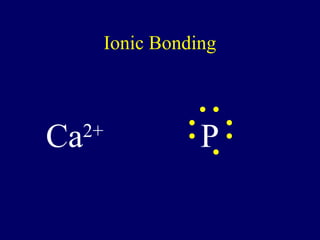
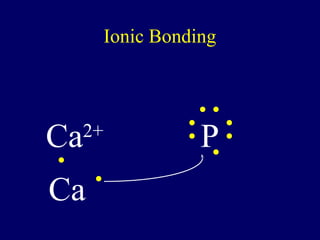
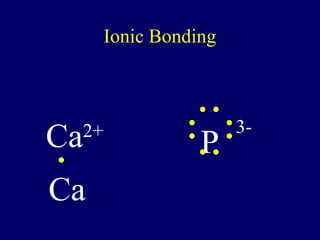
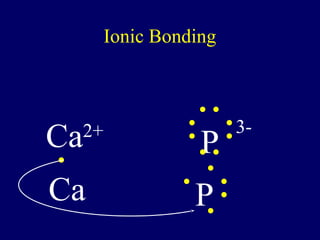
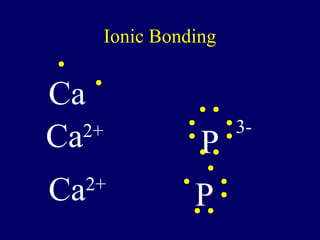
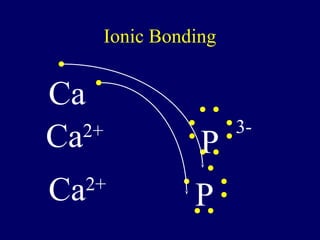
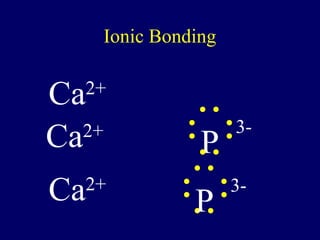
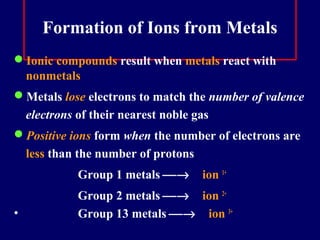
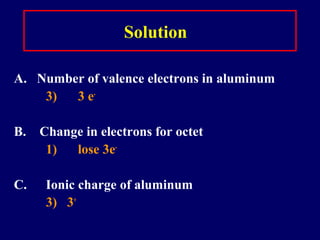
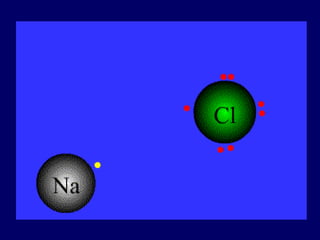
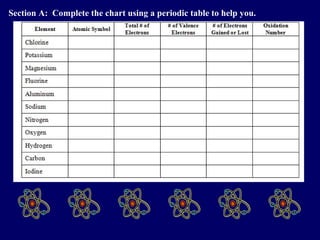
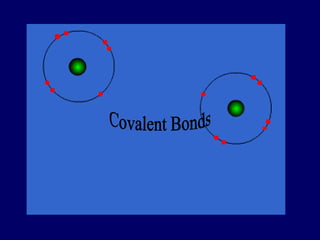
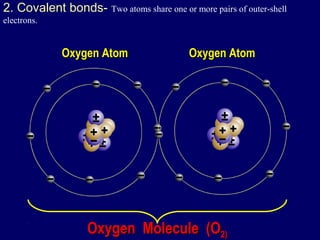
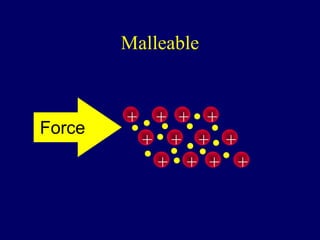
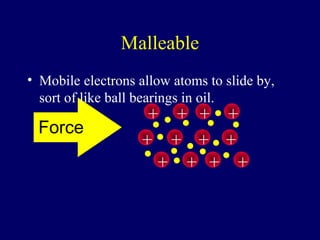
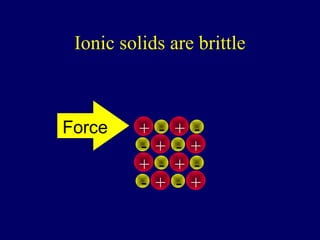
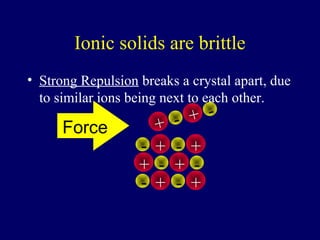
The document outlines the formation and properties of ionic compounds, explaining the octet rule, cation and anion formation, and the electrical charges associated. It describes the bonding process between metals and nonmetals, the characteristics of ionic solids, and introduces covalent and metallic bonds, including the significance of alloys. Additionally, it includes learning checks on valence electrons and ionic charges.