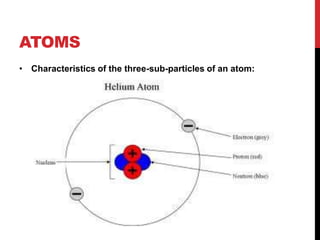
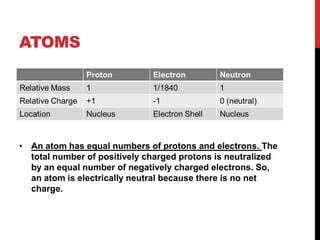
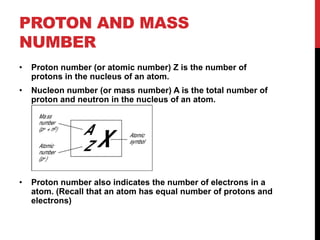
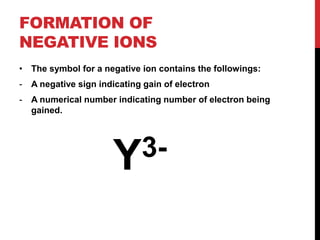
This document discusses atoms, ions, and molecules. It defines atoms as the smallest particle of an element that cannot be broken down further. Atoms are made up of protons, neutrons, and electrons. Ions are formed when atoms gain or lose electrons, giving them a positive or negative charge. The document also defines molecules as two or more atoms chemically bonded together, and can be made of the same or different elements. Chemical formulas indicate the types of elements and number of each atom in a substance.