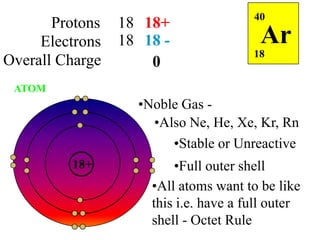
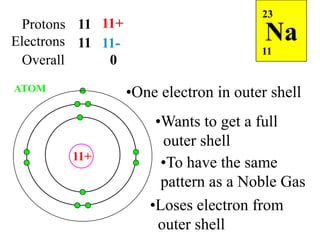
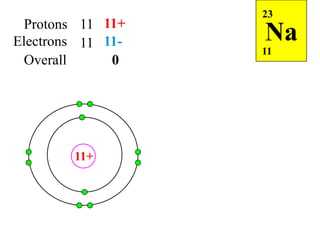
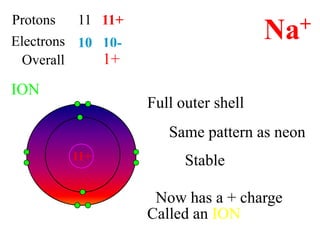
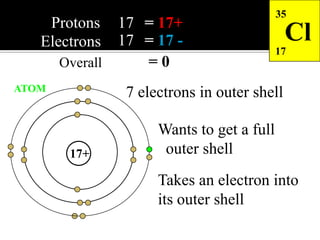
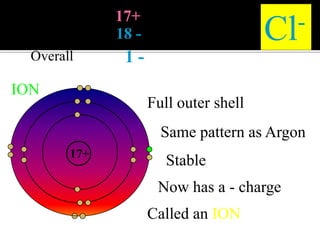
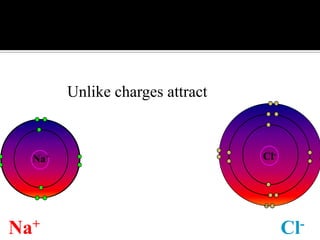
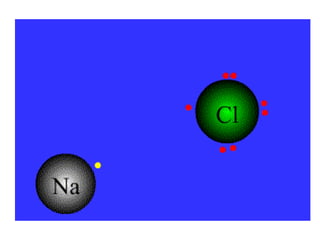
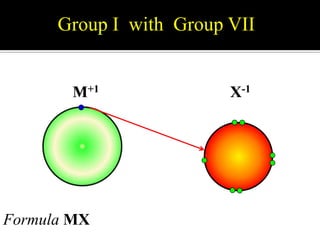
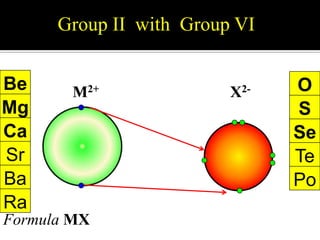
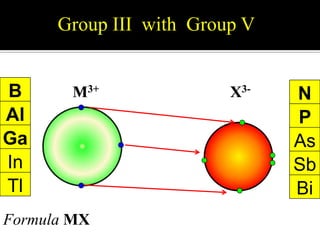
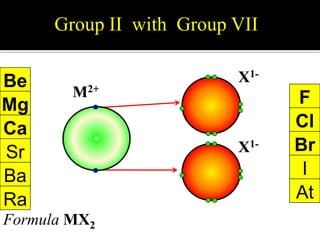
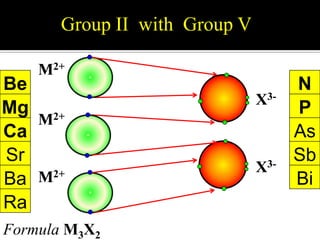
This document discusses ionic bonding, which involves the transfer of electrons between atoms to form ions. Metals form cations by losing electrons to achieve a full outer shell like a noble gas. Nonmetals form anions by gaining electrons to also achieve a full outer shell. Oppositely charged ions then attract through ionic bonding. The number of electrons lost or gained can be predicted based on the group of elements in the periodic table. Examples of ionic compounds formed between different groups are given along with their formulas and electron arrangements.




![ Atoms in the same group [column]
Have the same outer electron
configuration.
Have the same valence electrons.
Easily found by looking up the group
number on the periodic table.
Group 2A - Be, Mg, Ca, etc. 2 valence electrons](https://image.slidesharecdn.com/2-140309055131-phpapp02/85/2-2-1-ionic-bonding_form_v-1-13-320.jpg)

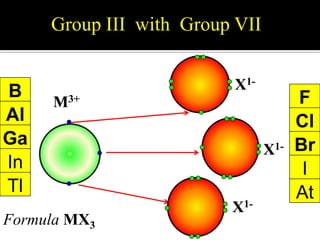
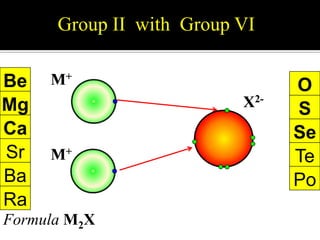
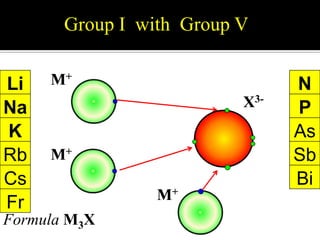
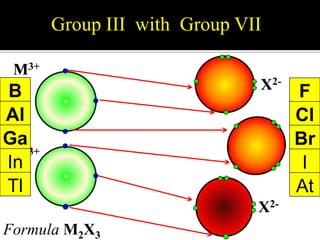
![
Aluminium :oxygen •
Magnesium :nitrogen
Barium: Arsenic
•
Aluminium :Iodine
Gallium :Sulphur
•
Barium :Oxygen
Sodium :Fluorine
•
Draw diagrams – outer
shell only required
Show electron movement
with arrows
Show atoms with charge
and number
Write formula [without
charges] and name](https://image.slidesharecdn.com/2-140309055131-phpapp02/85/2-2-1-ionic-bonding_form_v-1-24-320.jpg)