Embed presentation
Downloaded 16 times

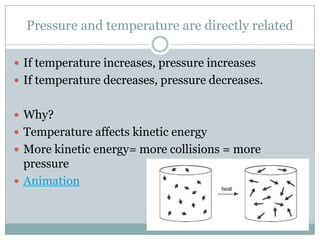

Gay-Lussac's Law states that pressure and temperature are directly related for a gas at constant volume: if temperature increases, pressure increases; if temperature decreases, pressure decreases. This is because temperature affects the kinetic energy of gas particles - higher temperature means more kinetic energy and more frequent and energetic particle collisions, resulting in higher pressure. The law can be expressed as P1/T1 = P2/T2, allowing calculations of pressure changes with temperature changes when volume is held constant and temperatures are in Kelvin.


