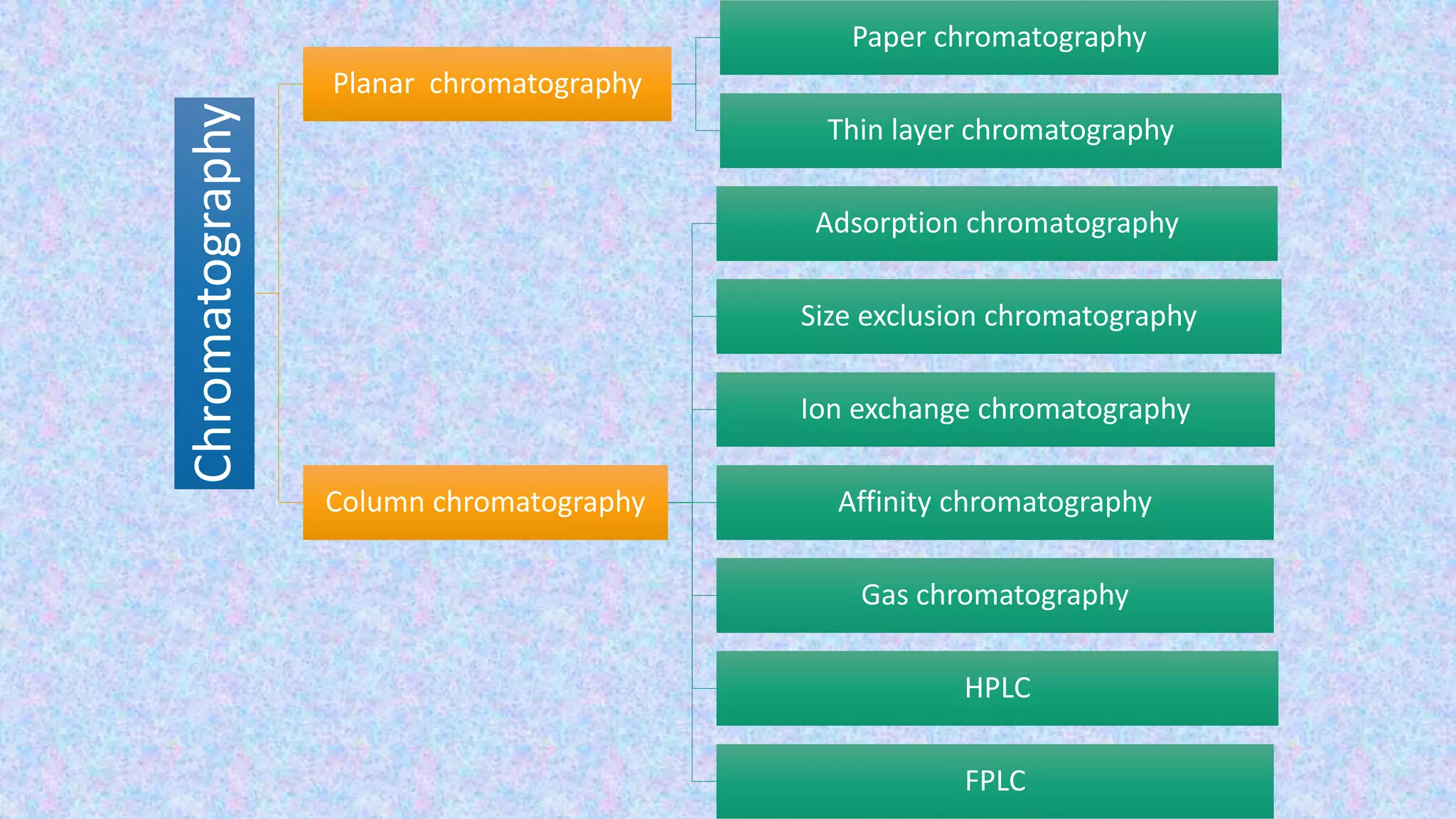
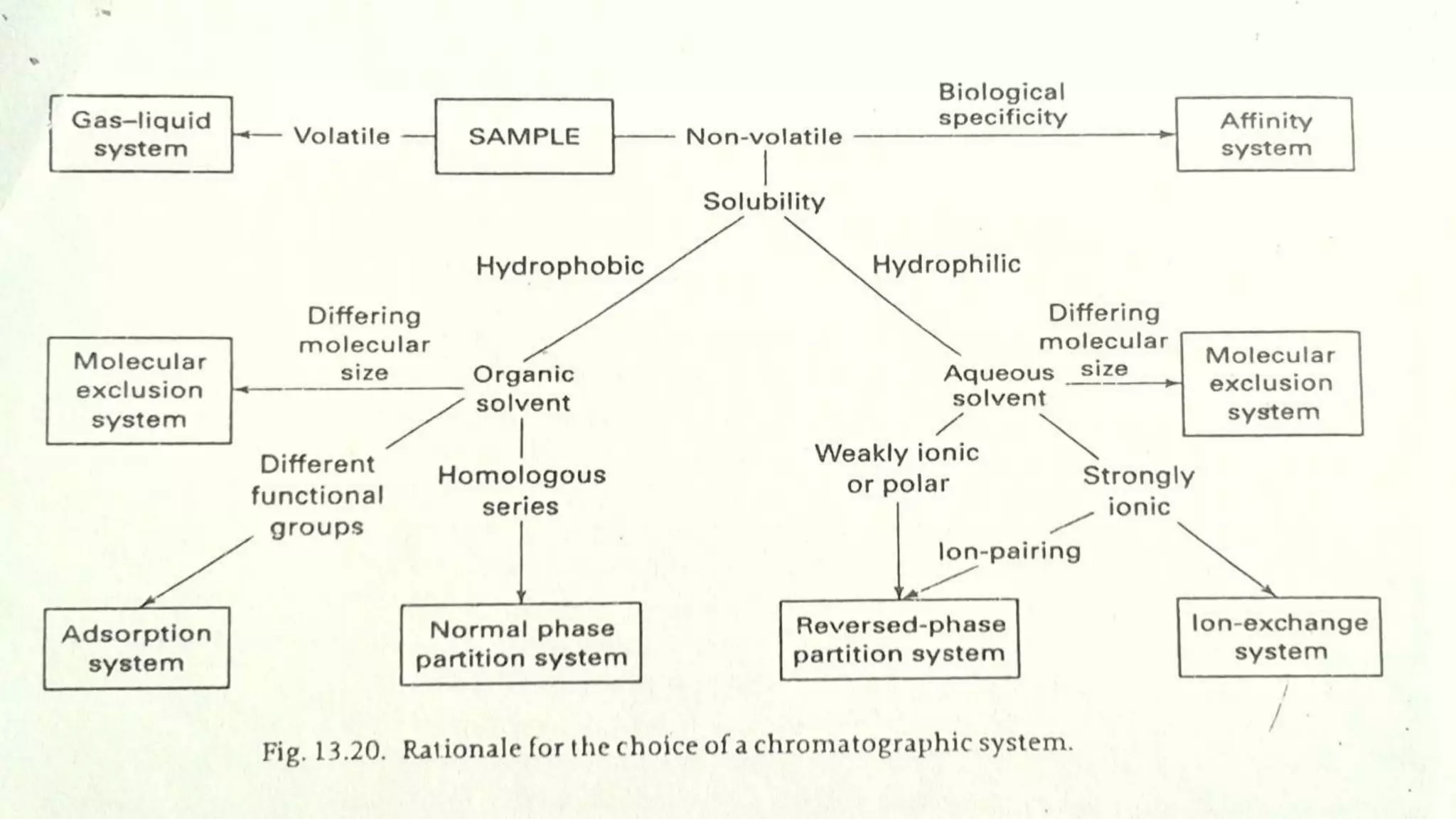
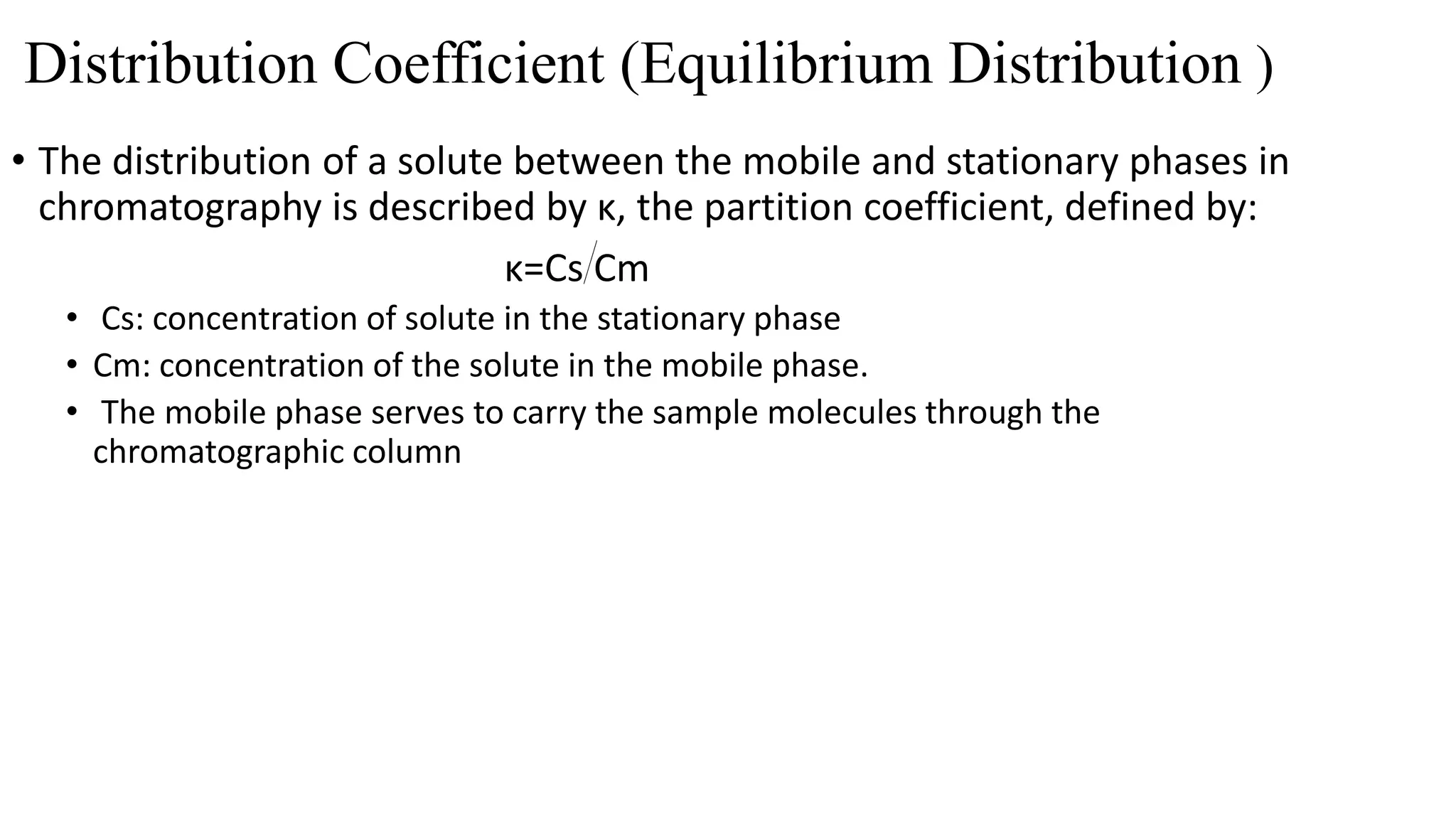
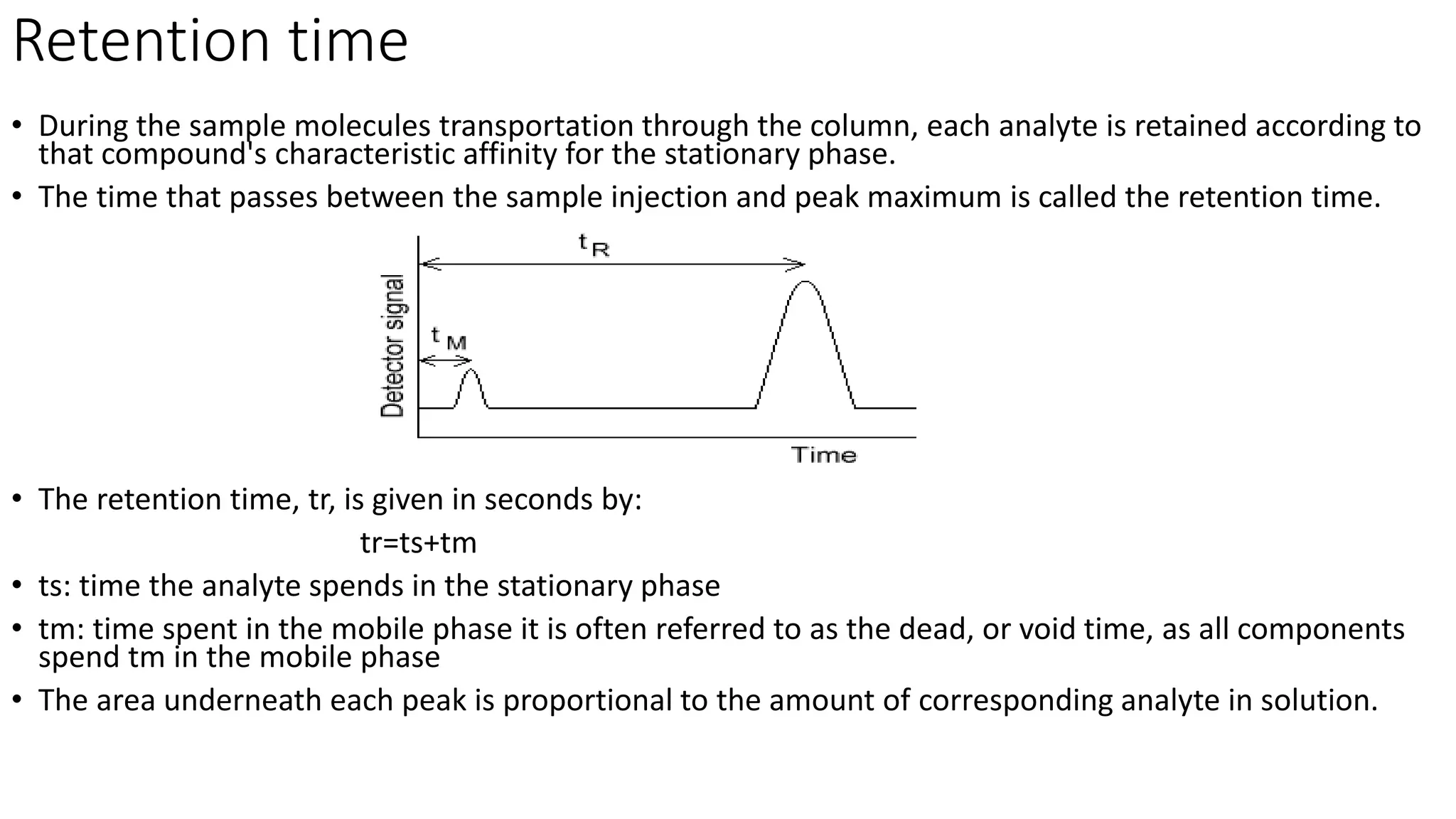
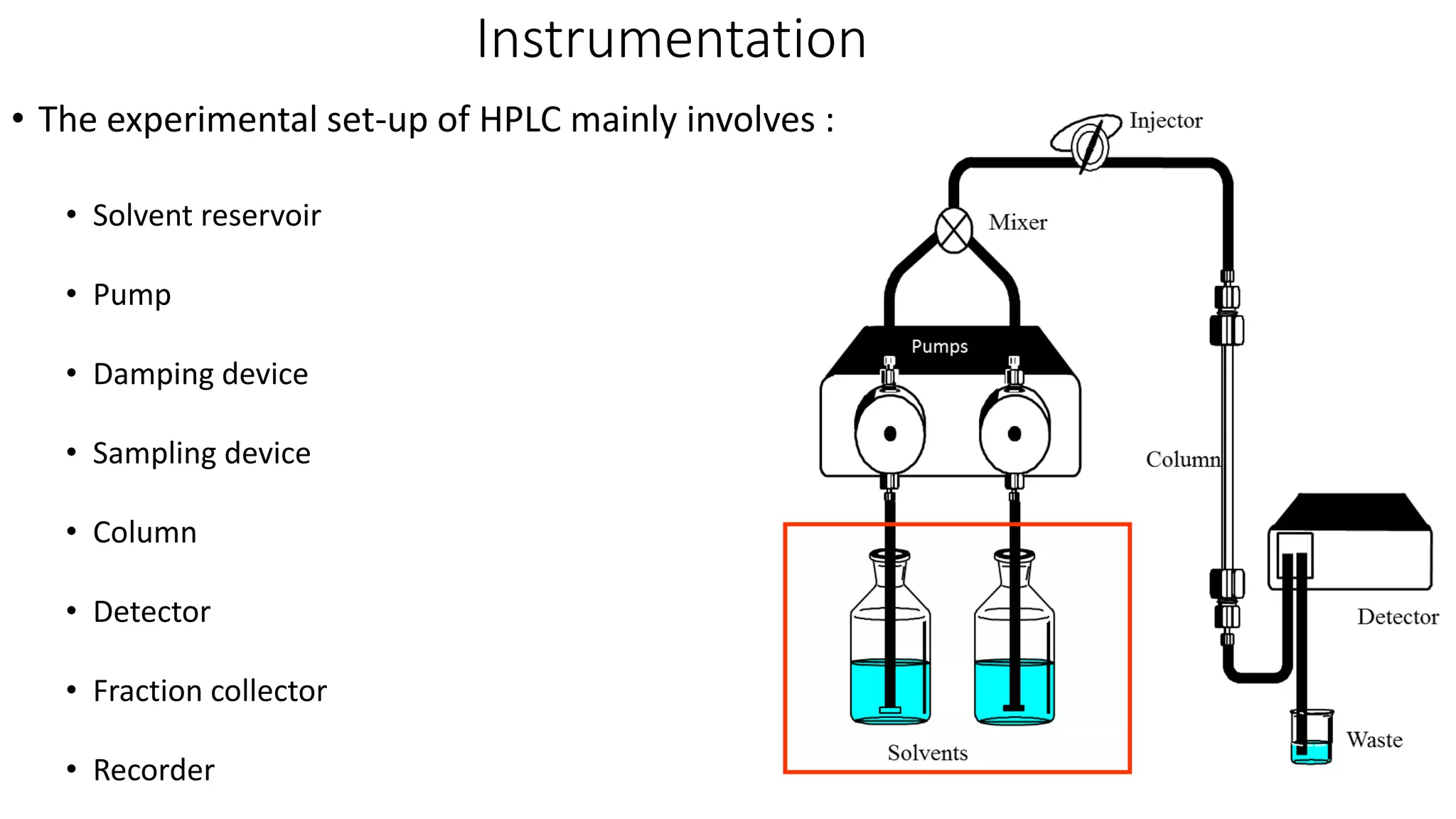
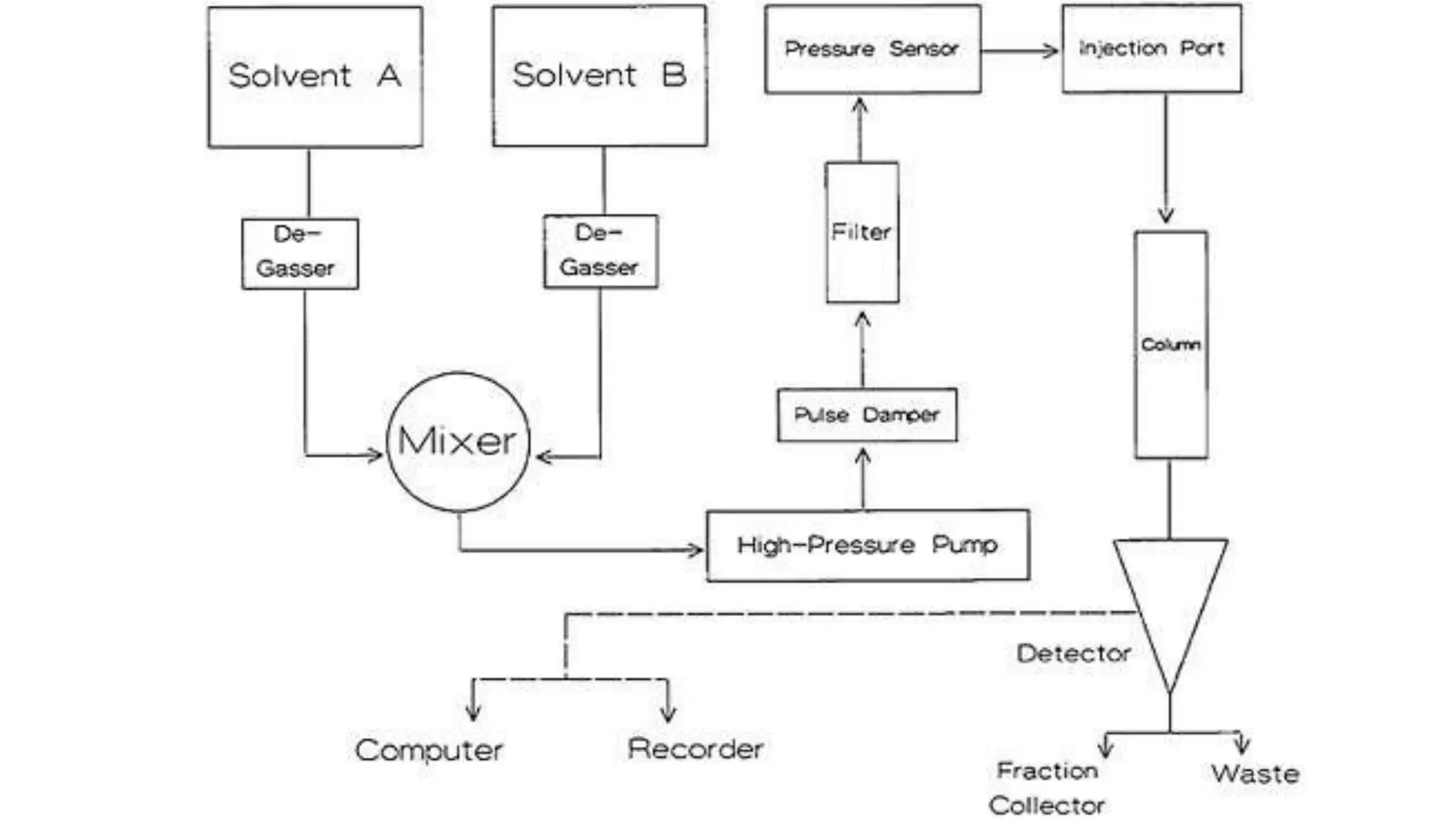
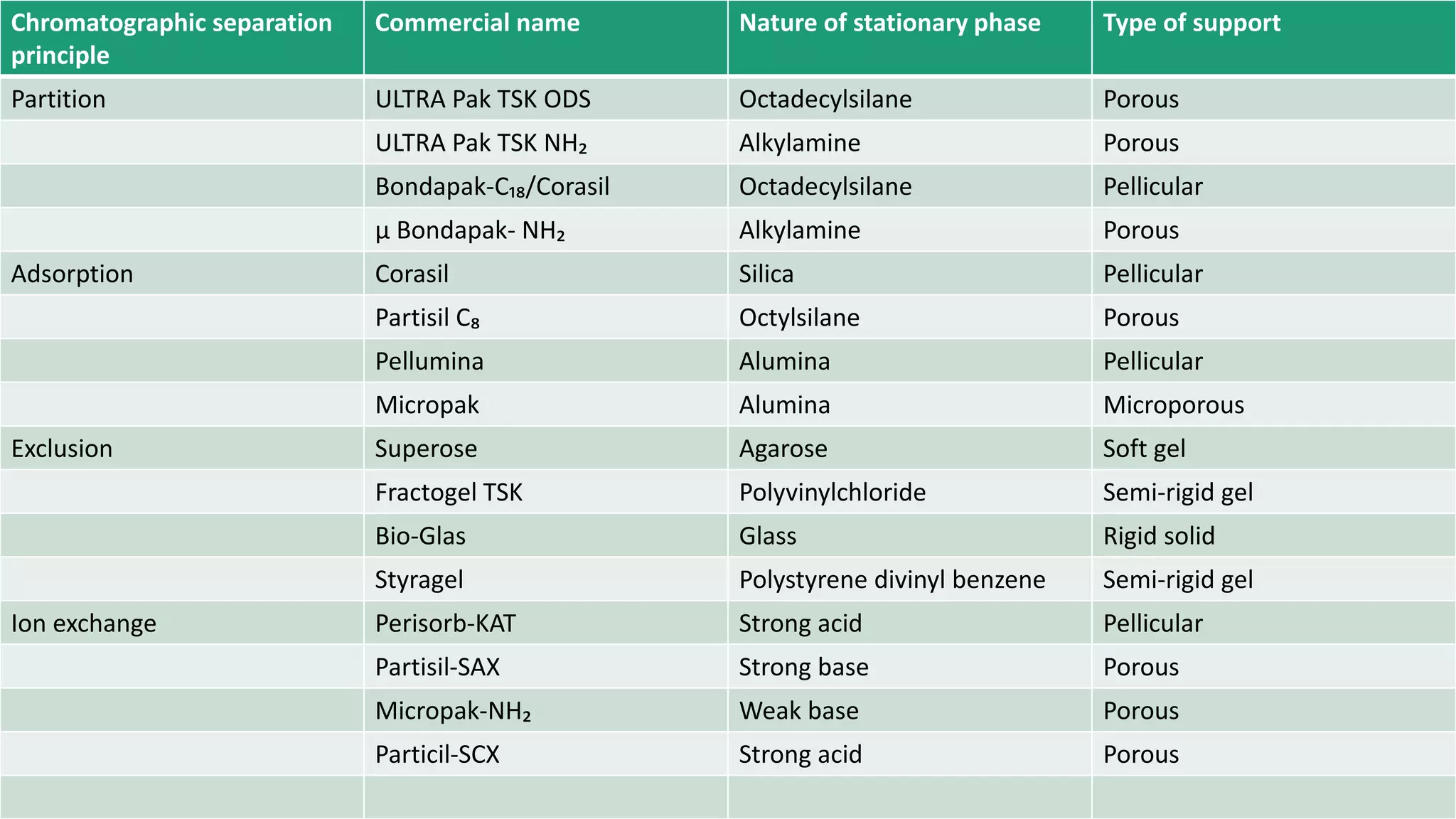
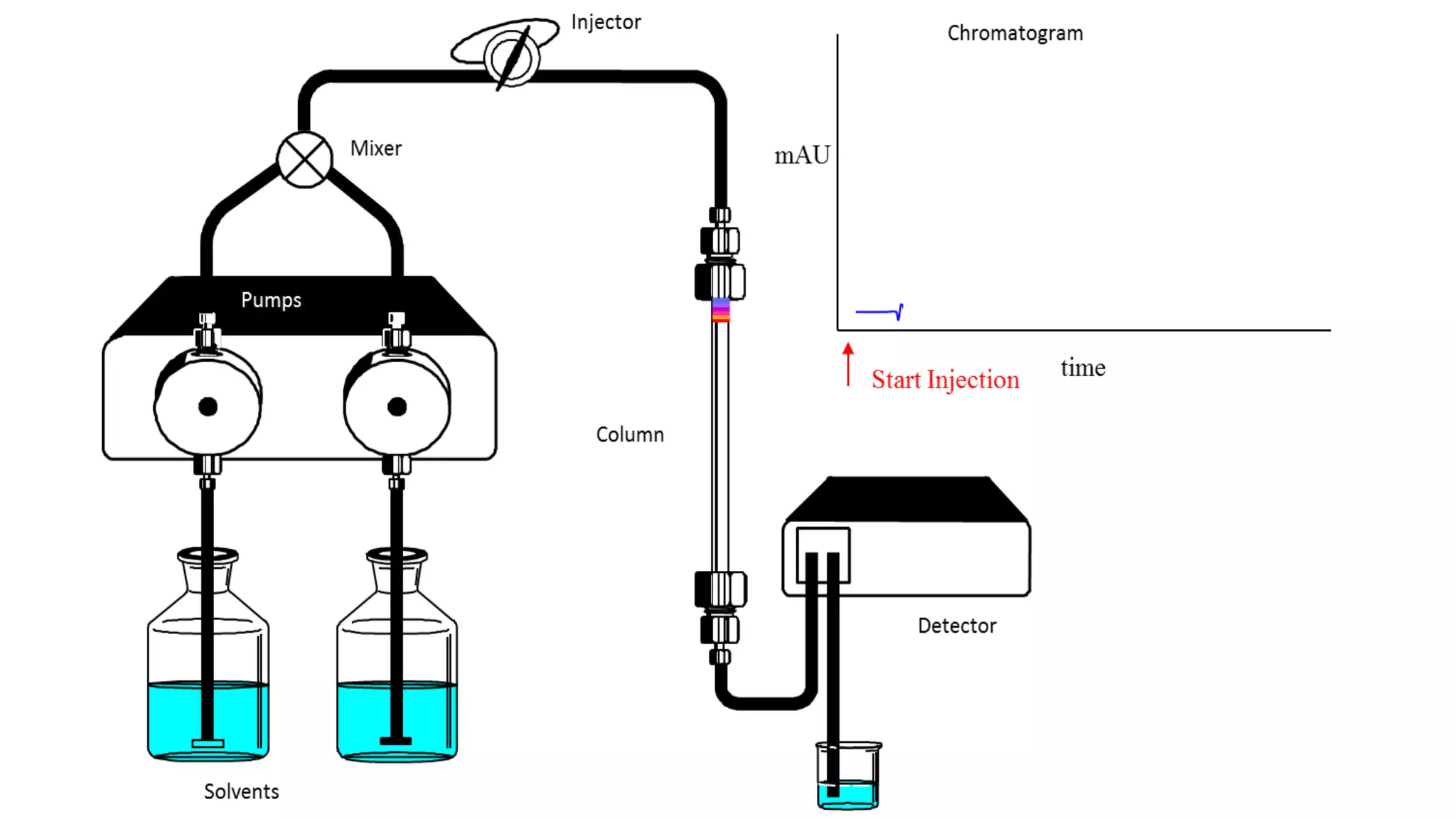
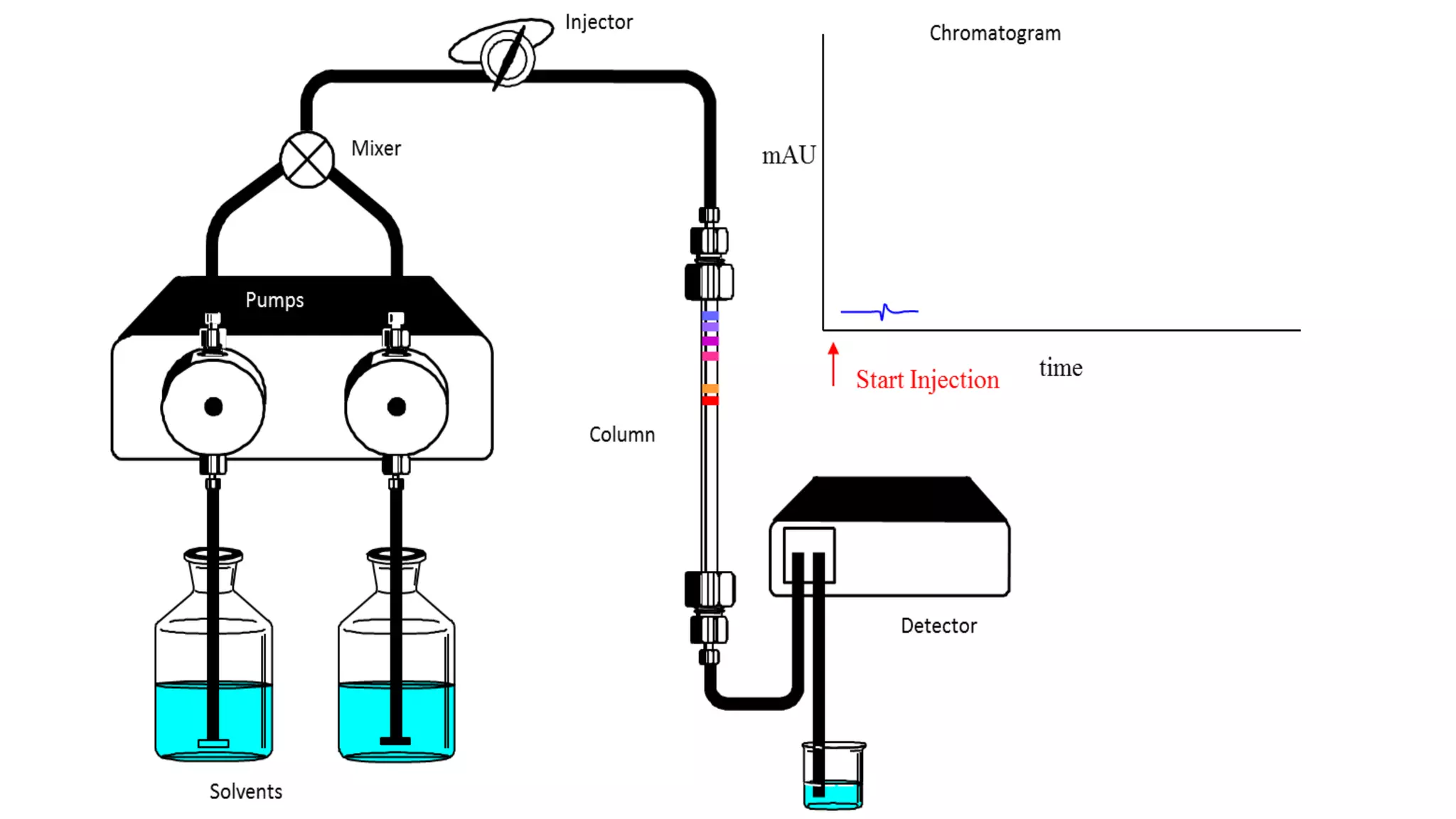
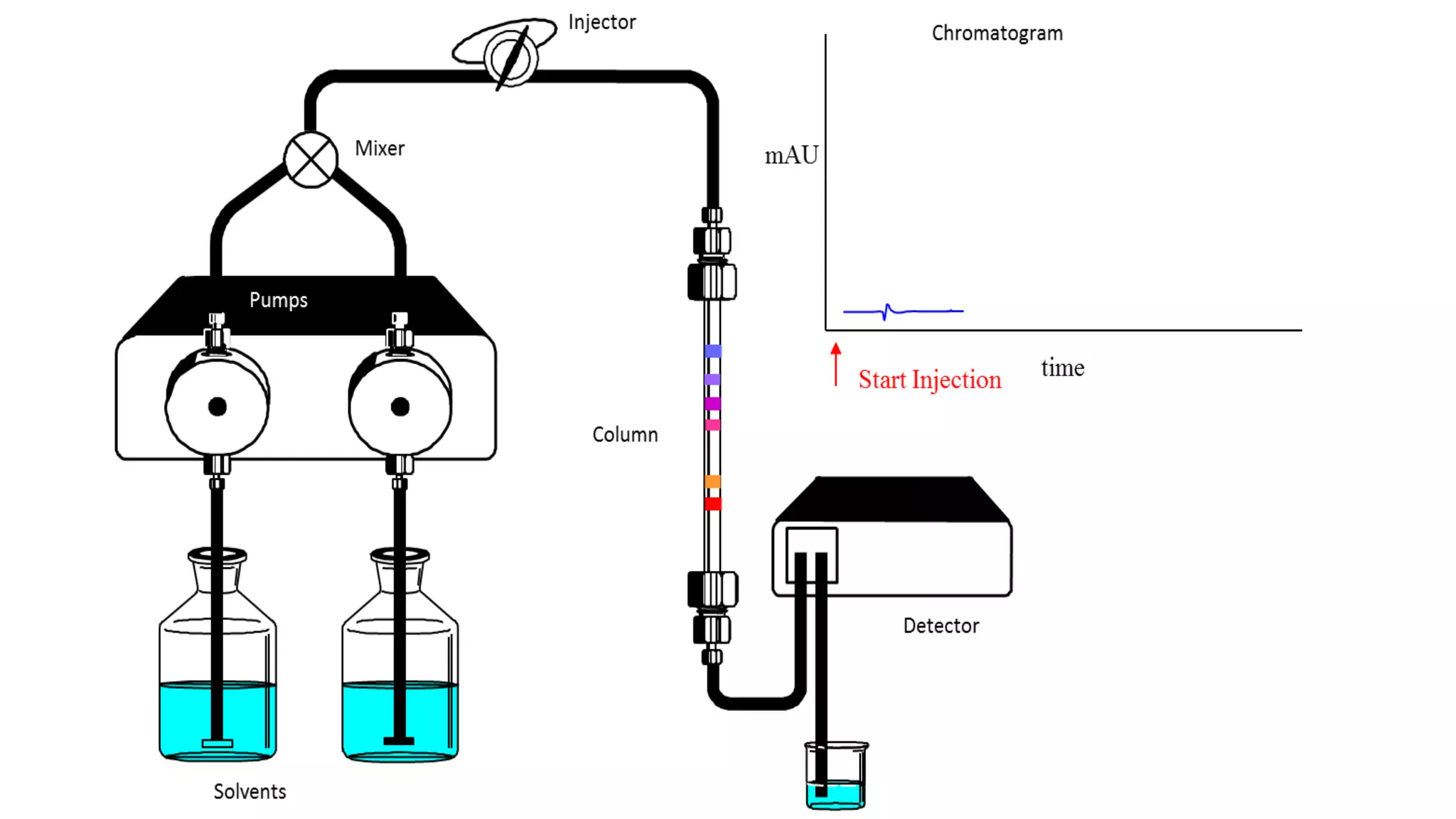
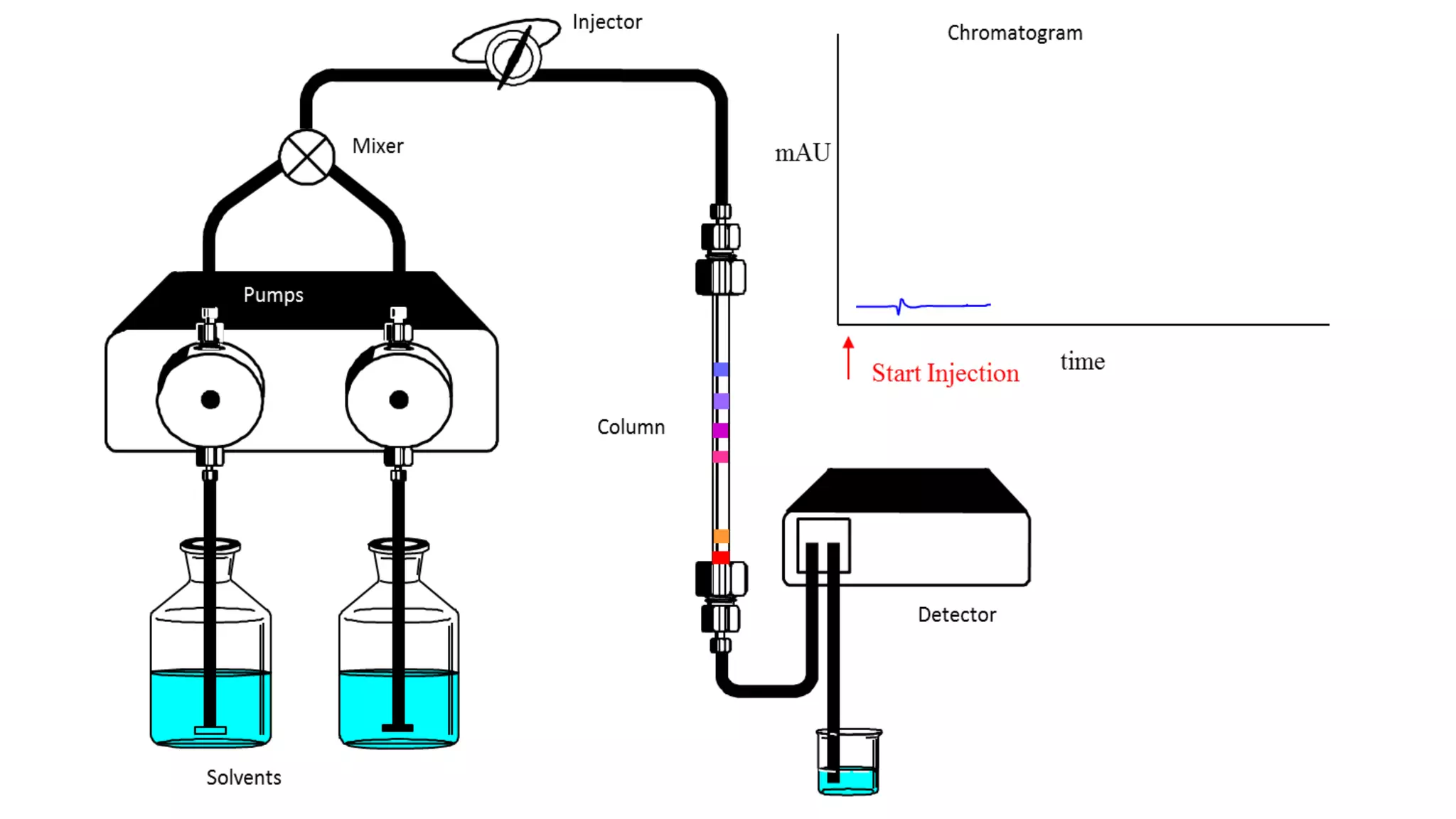
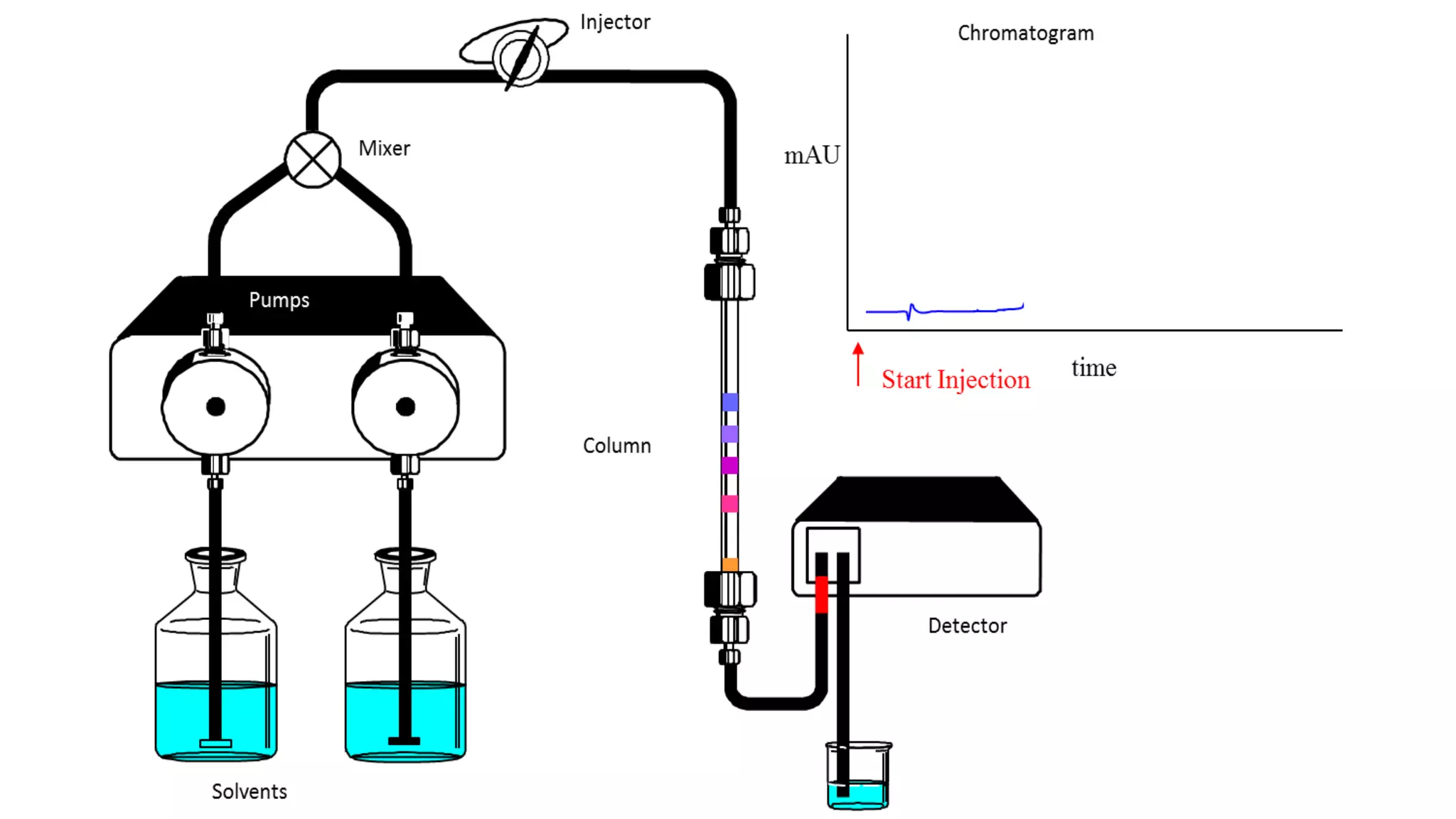
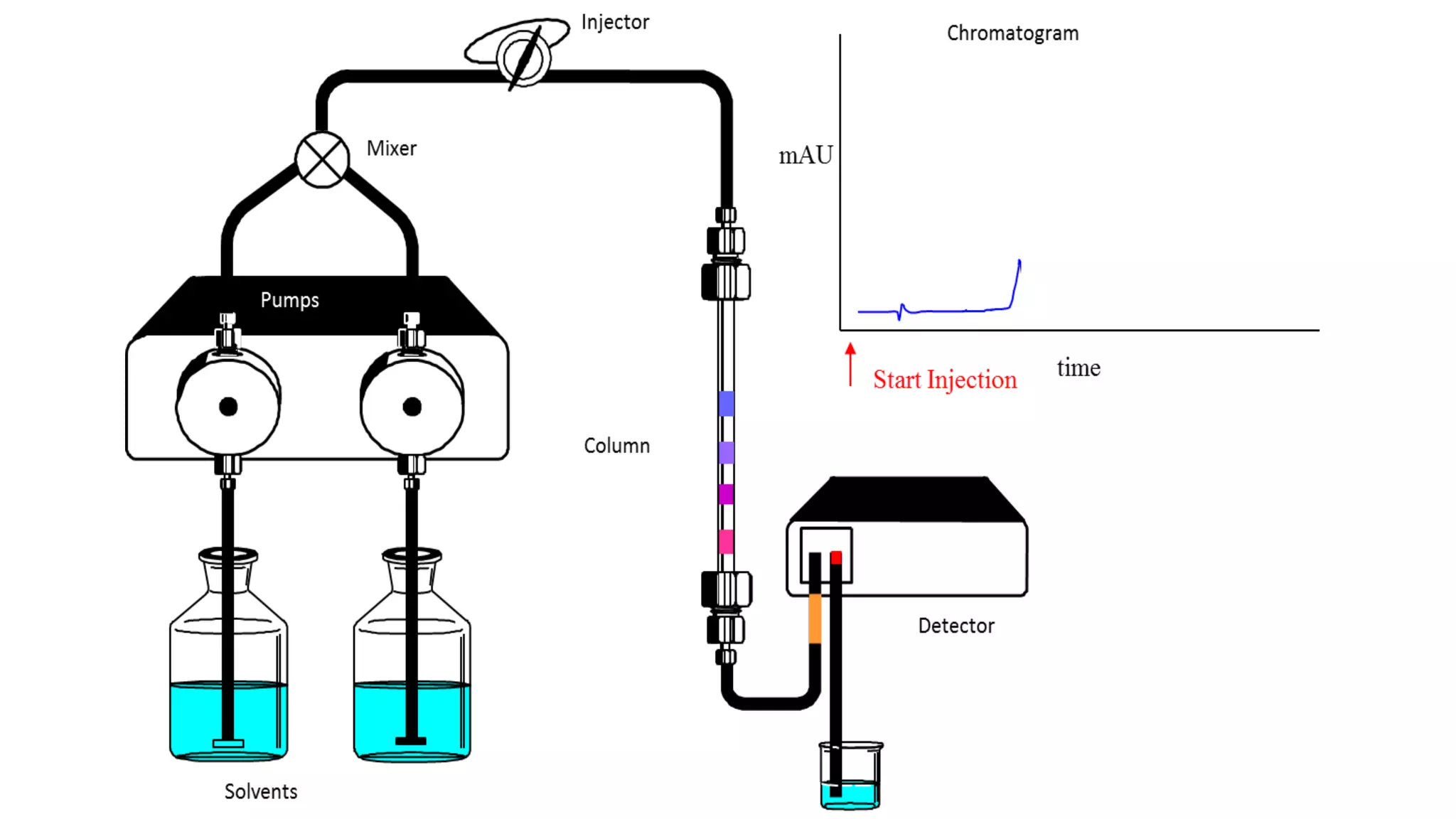
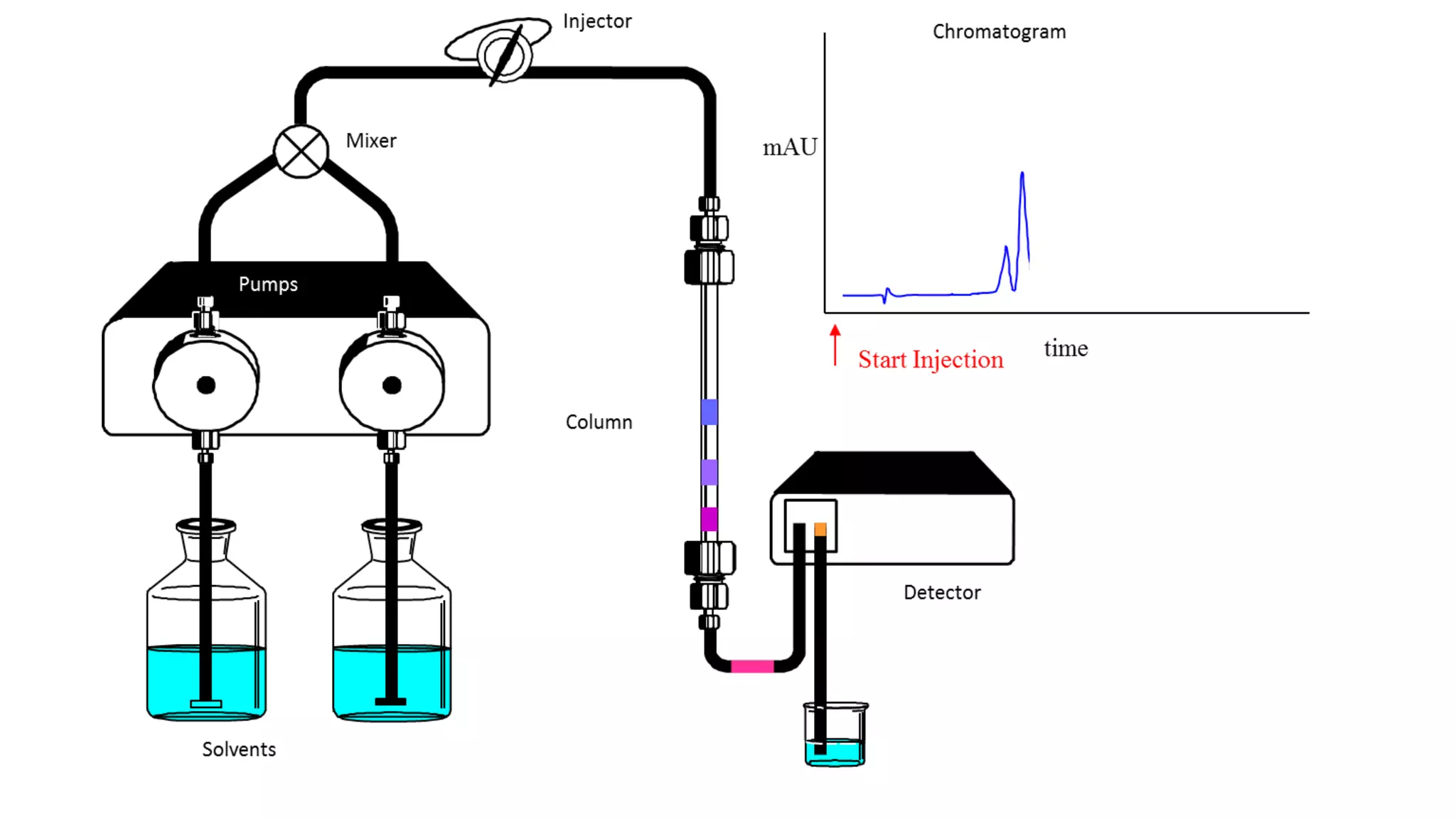
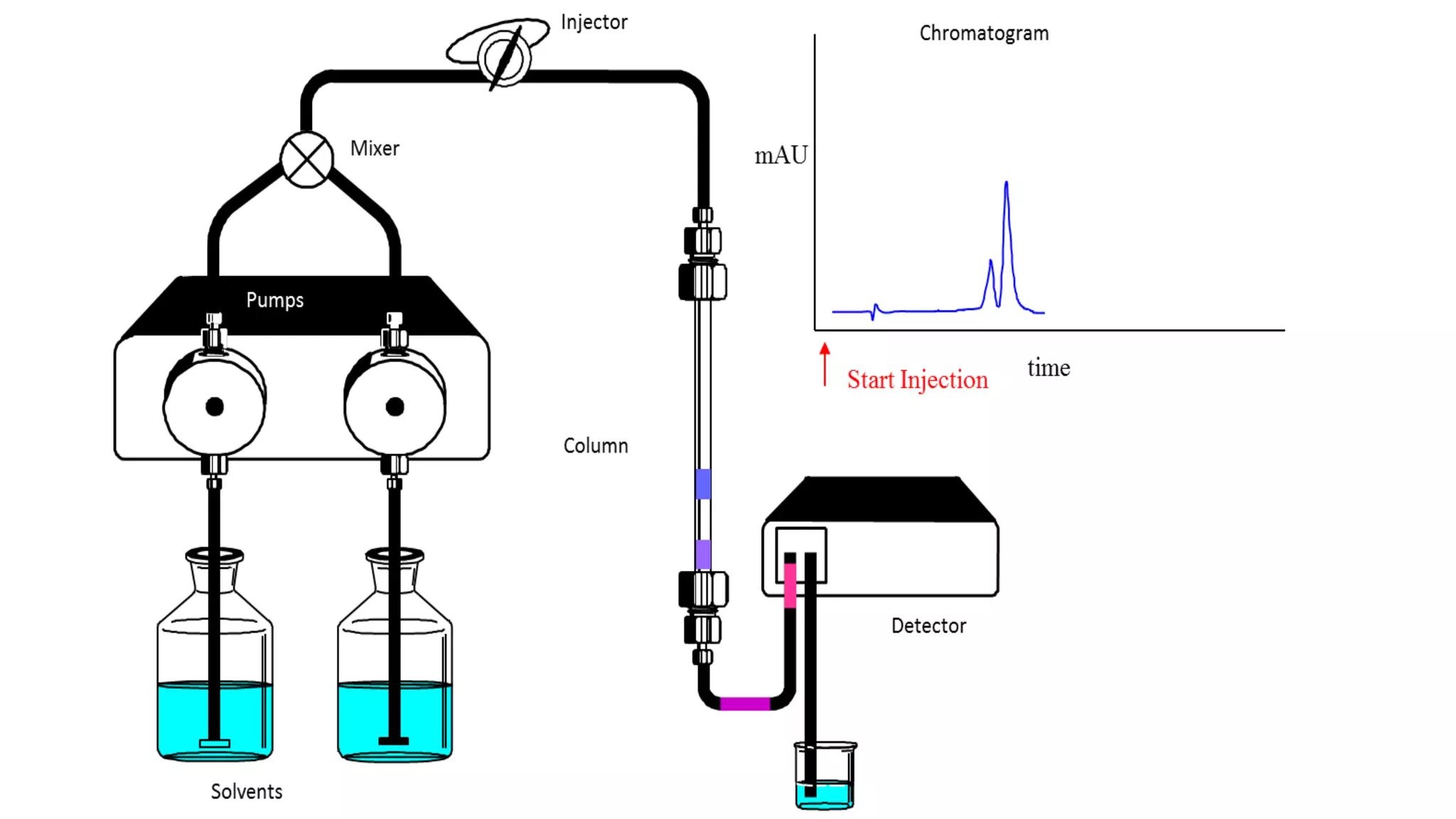
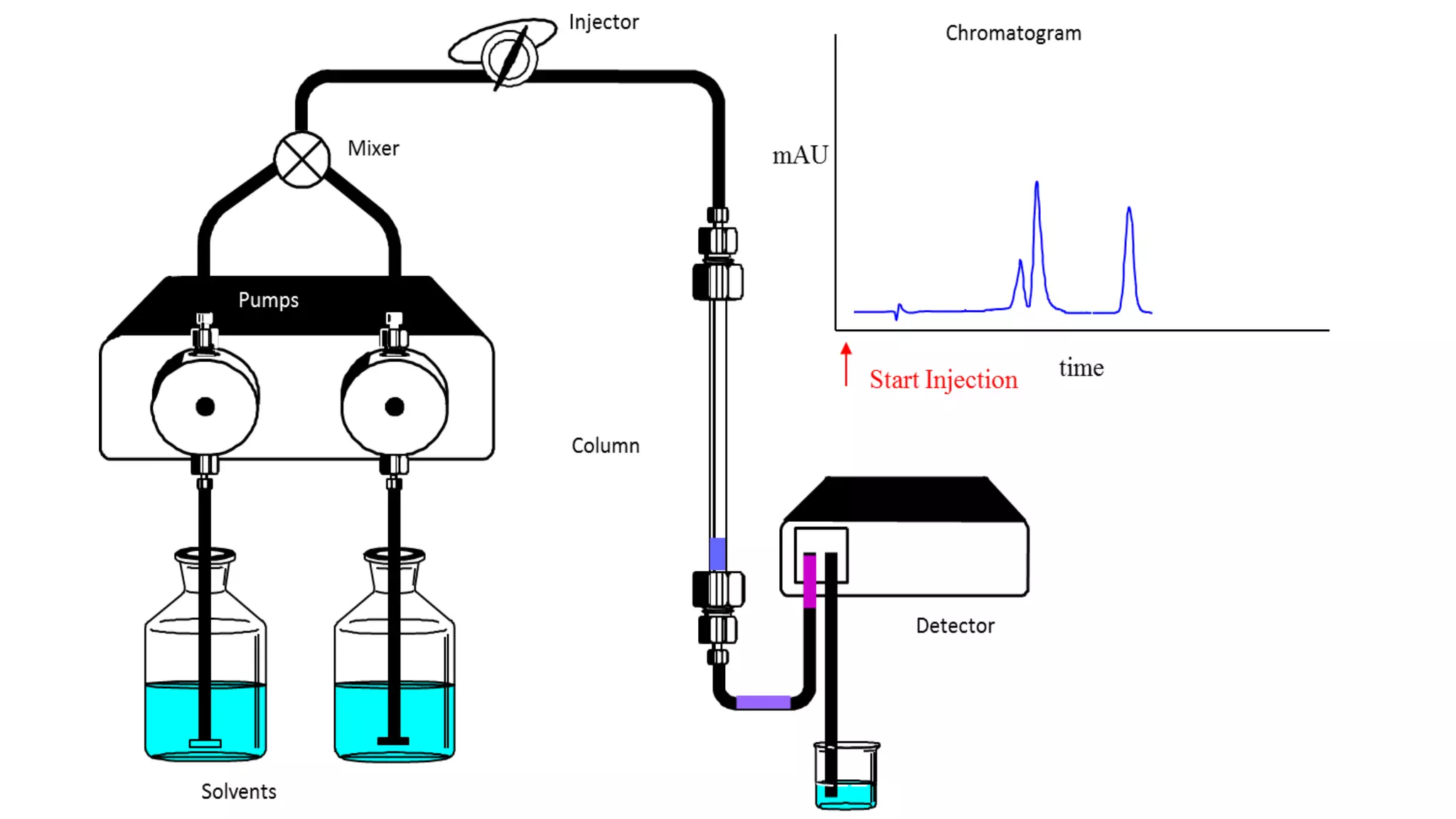
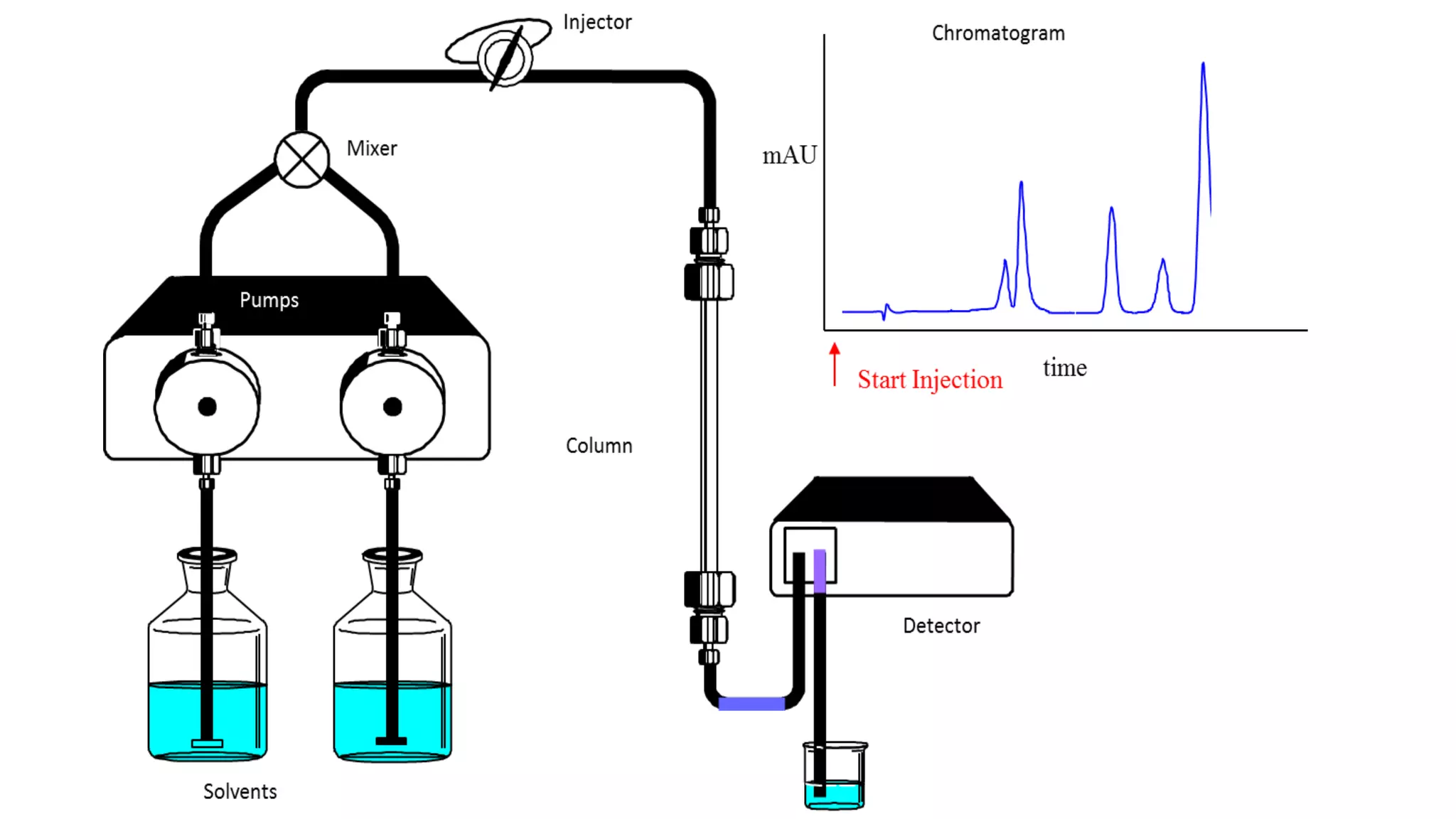
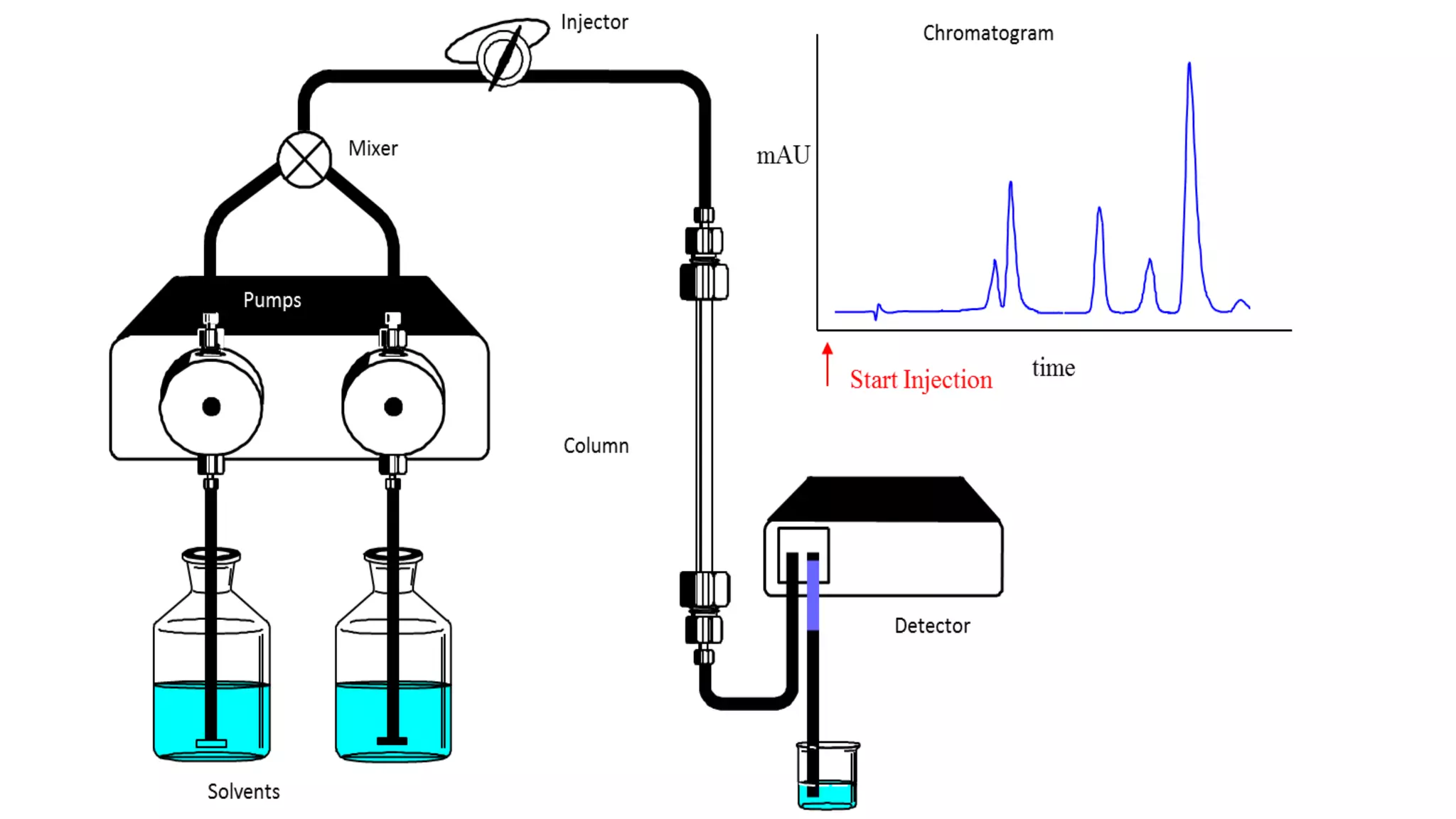
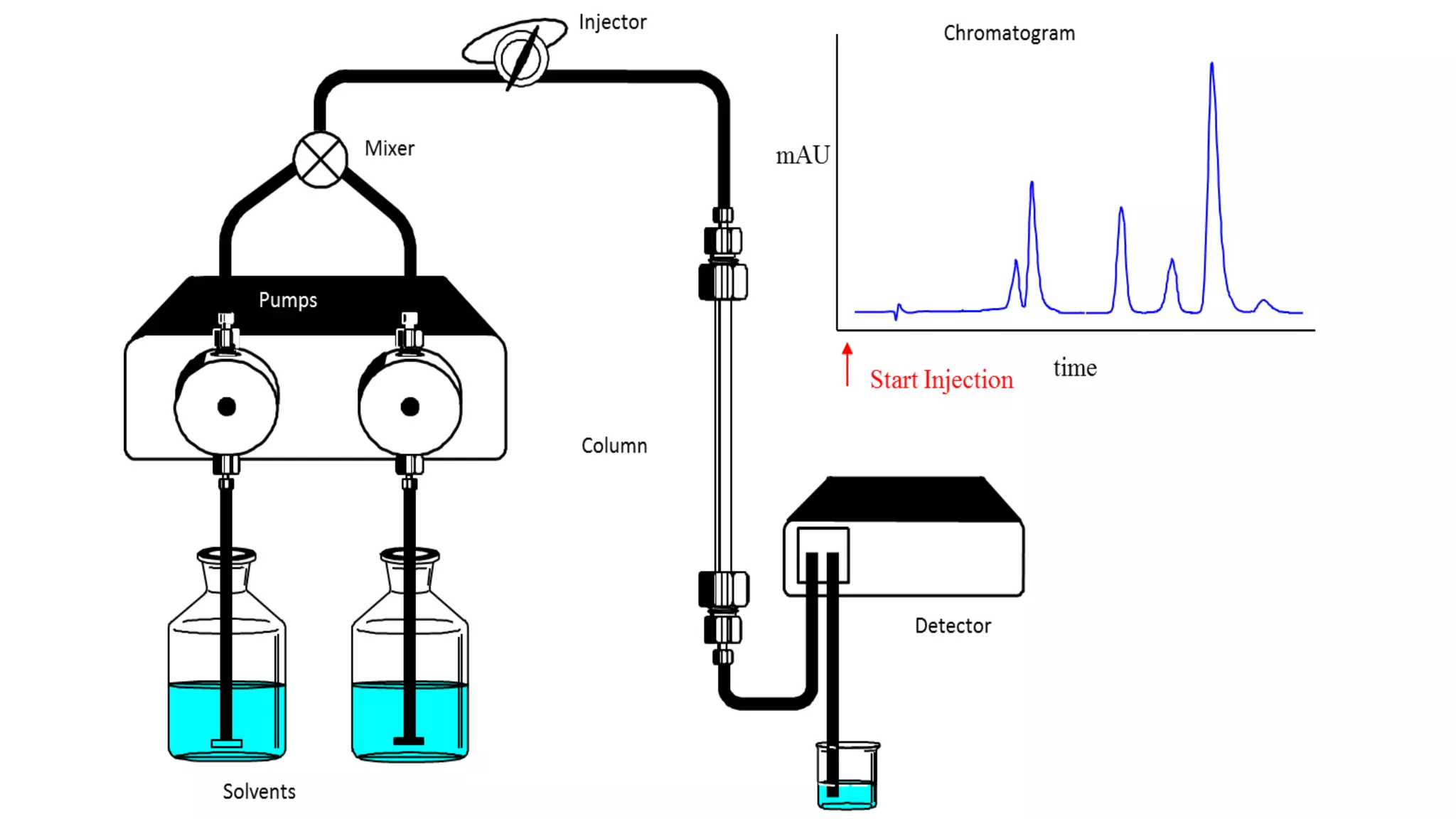
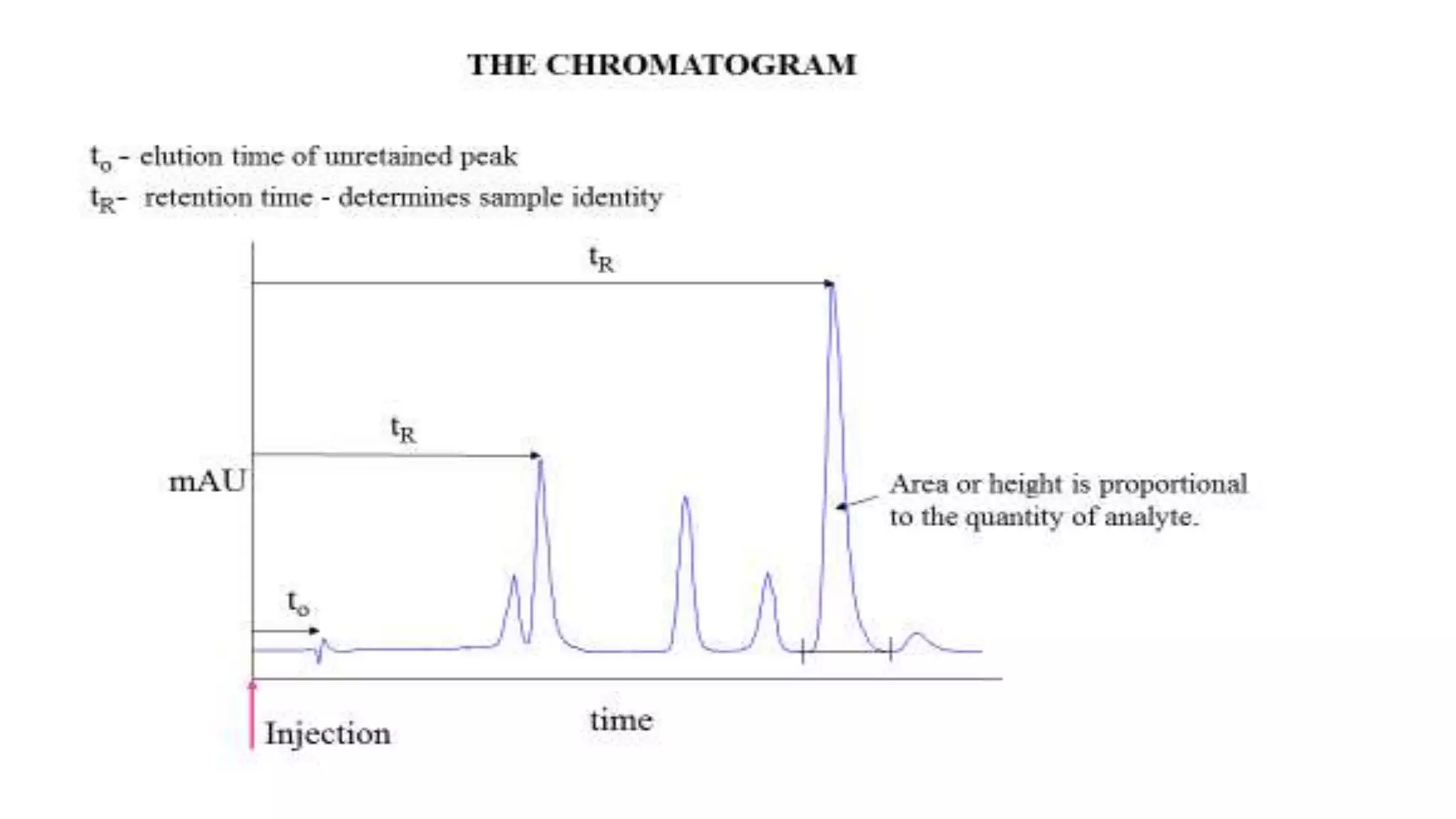
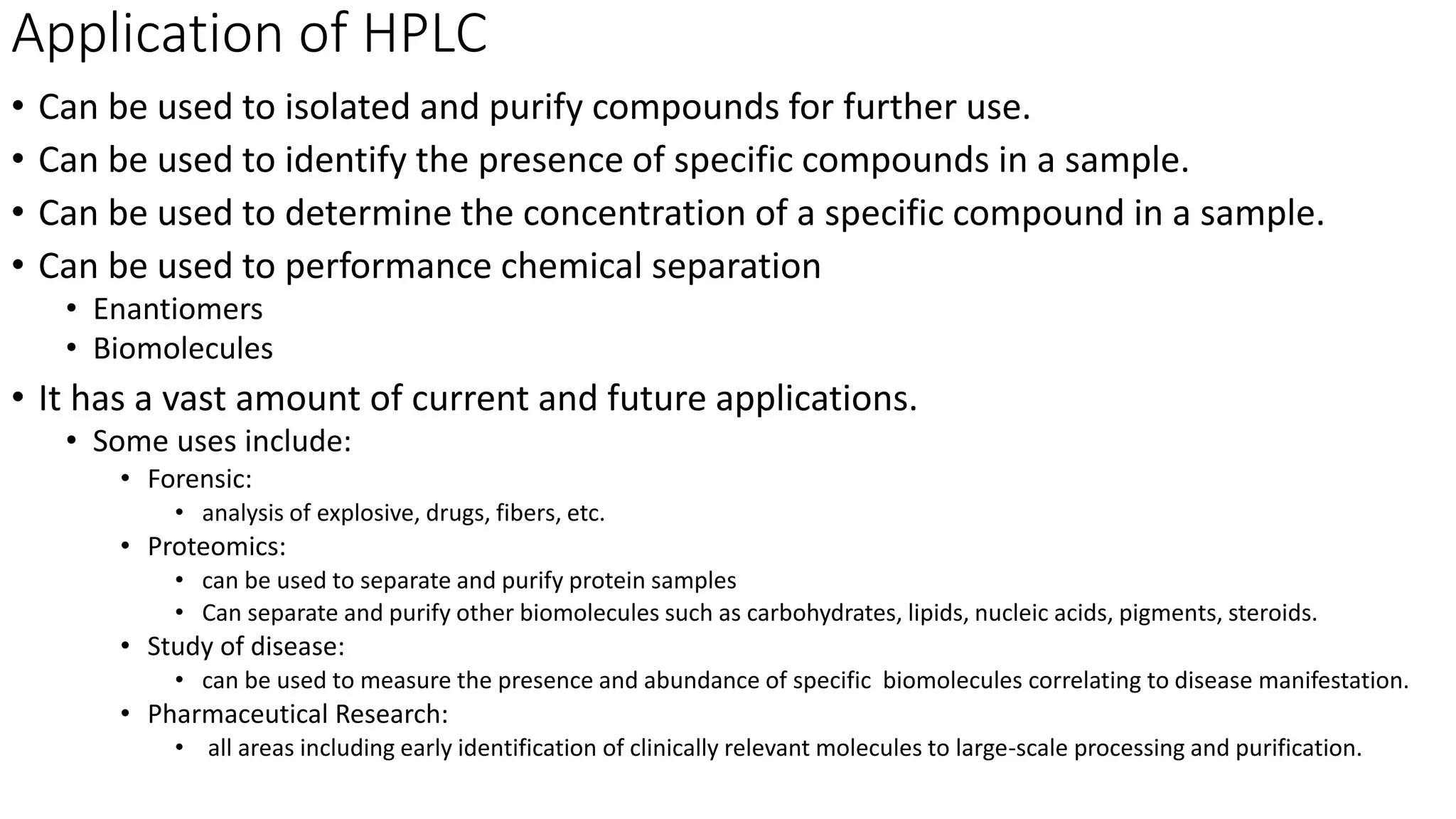
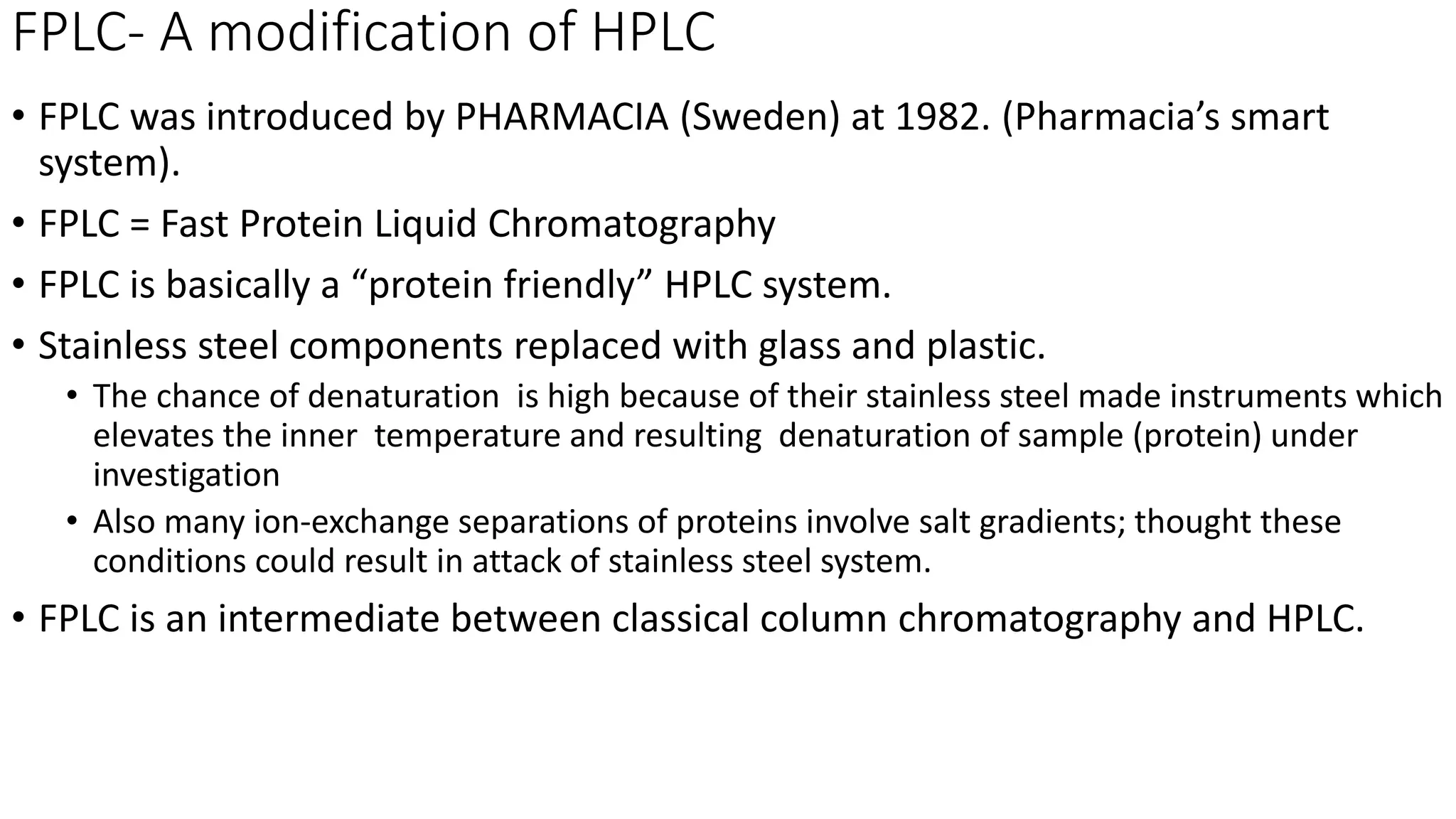
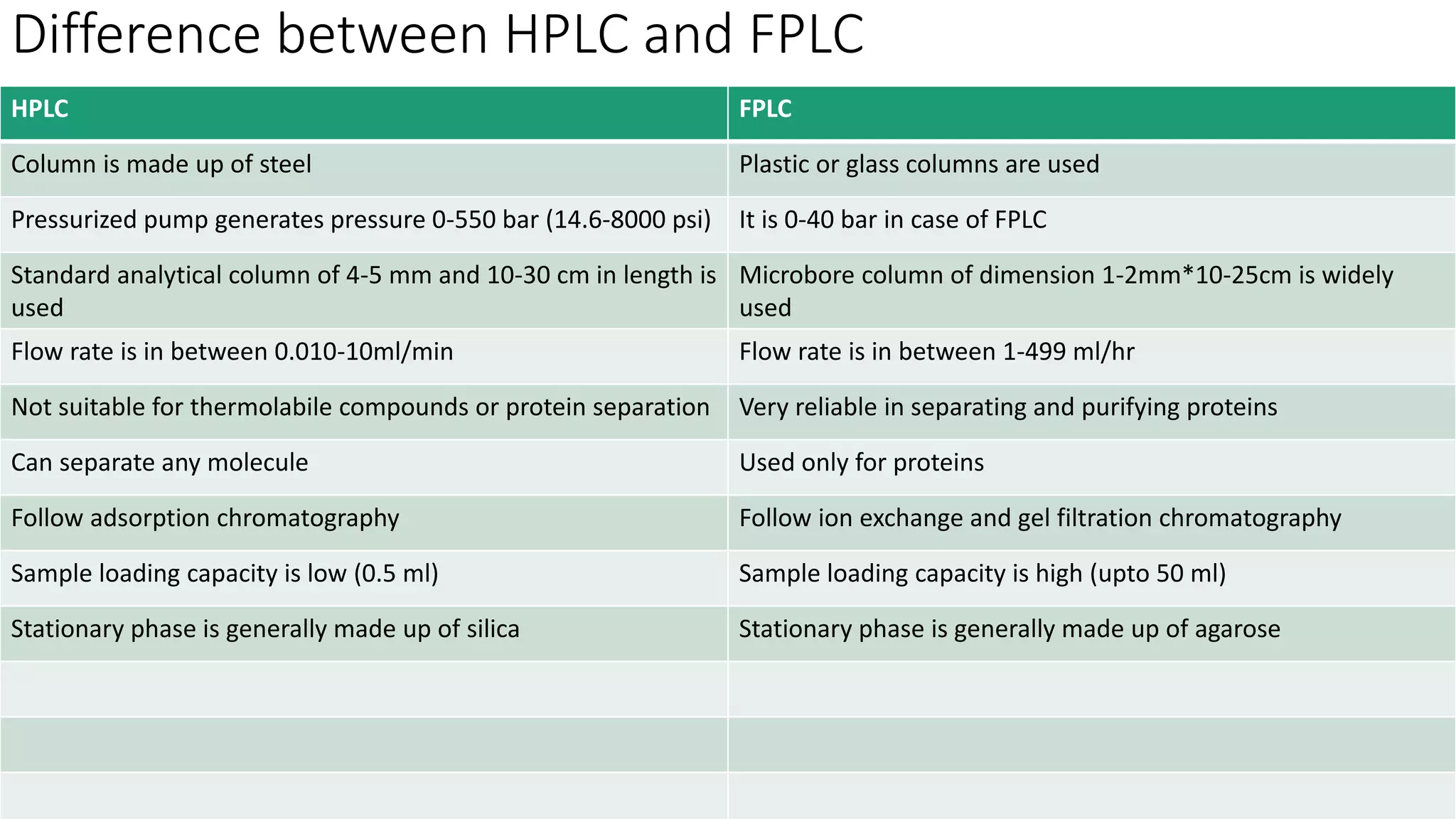
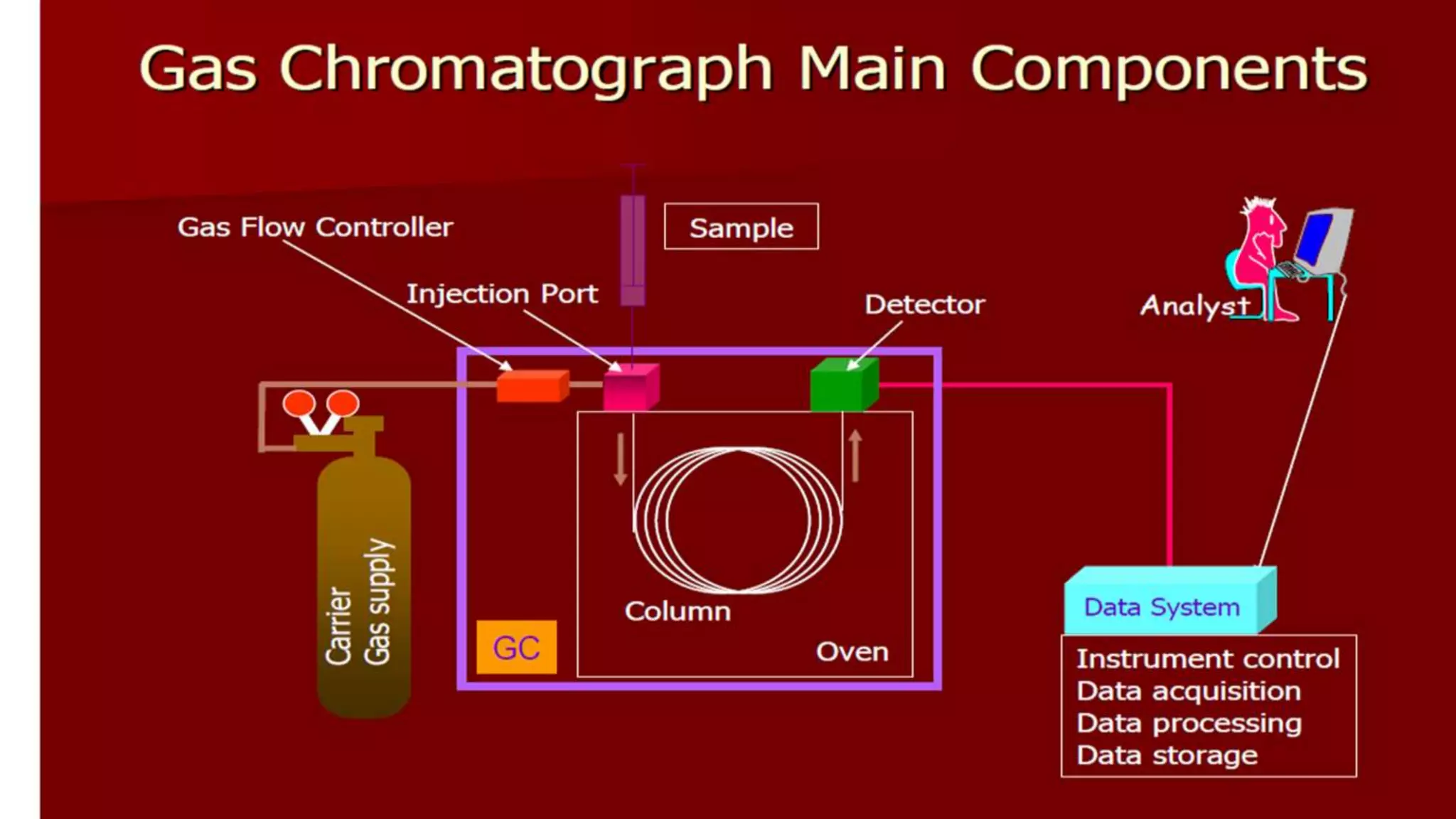
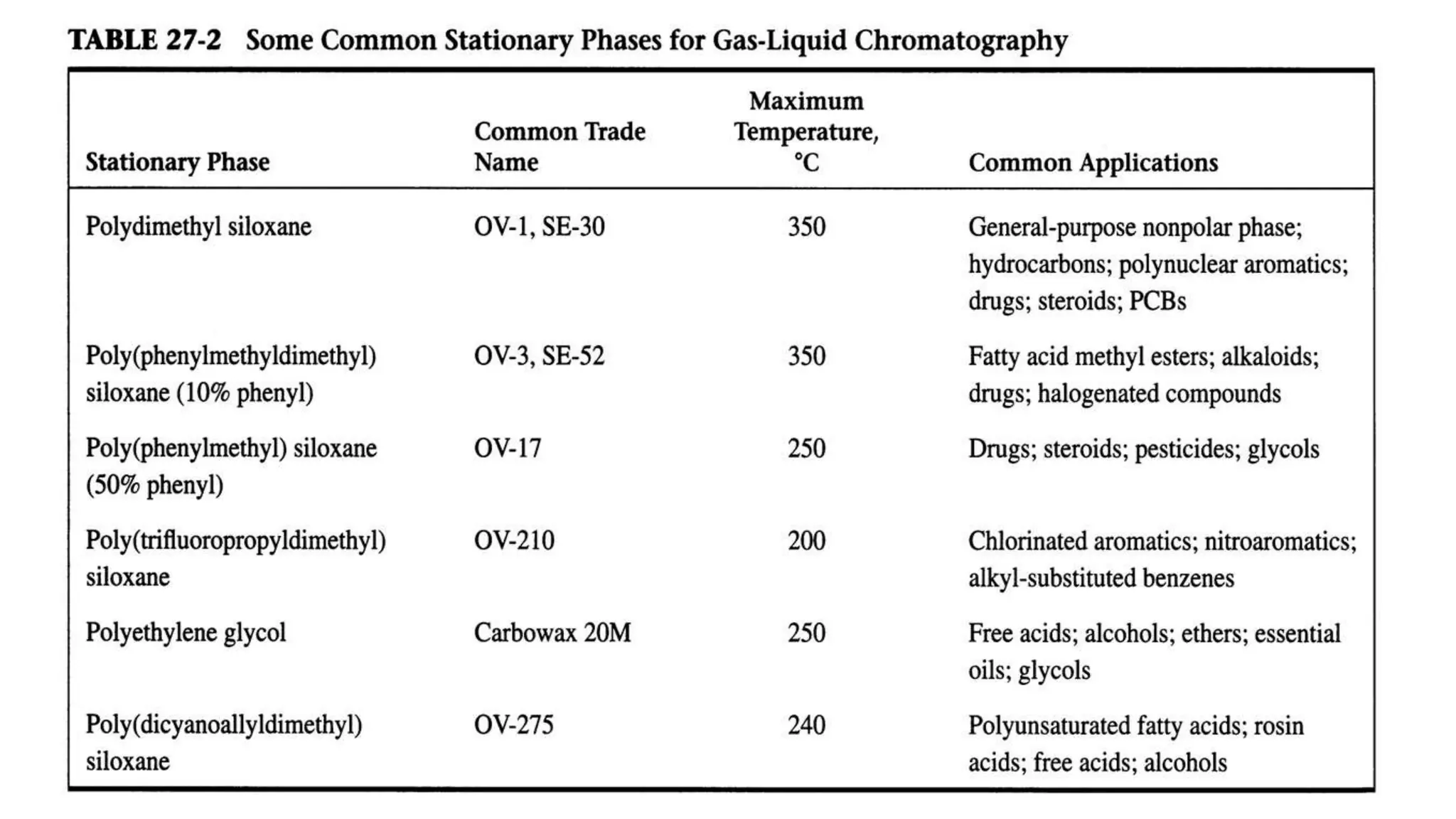
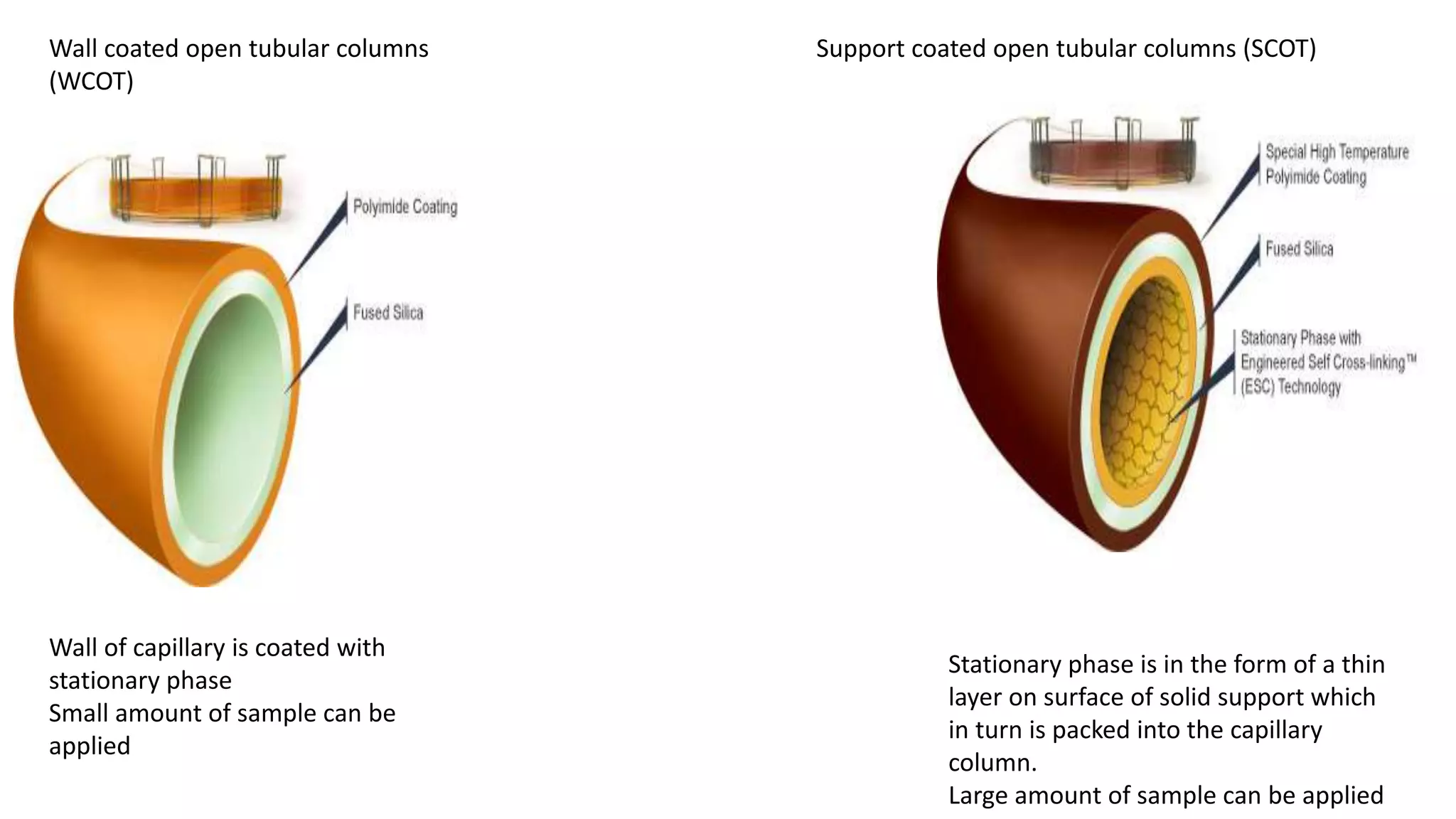
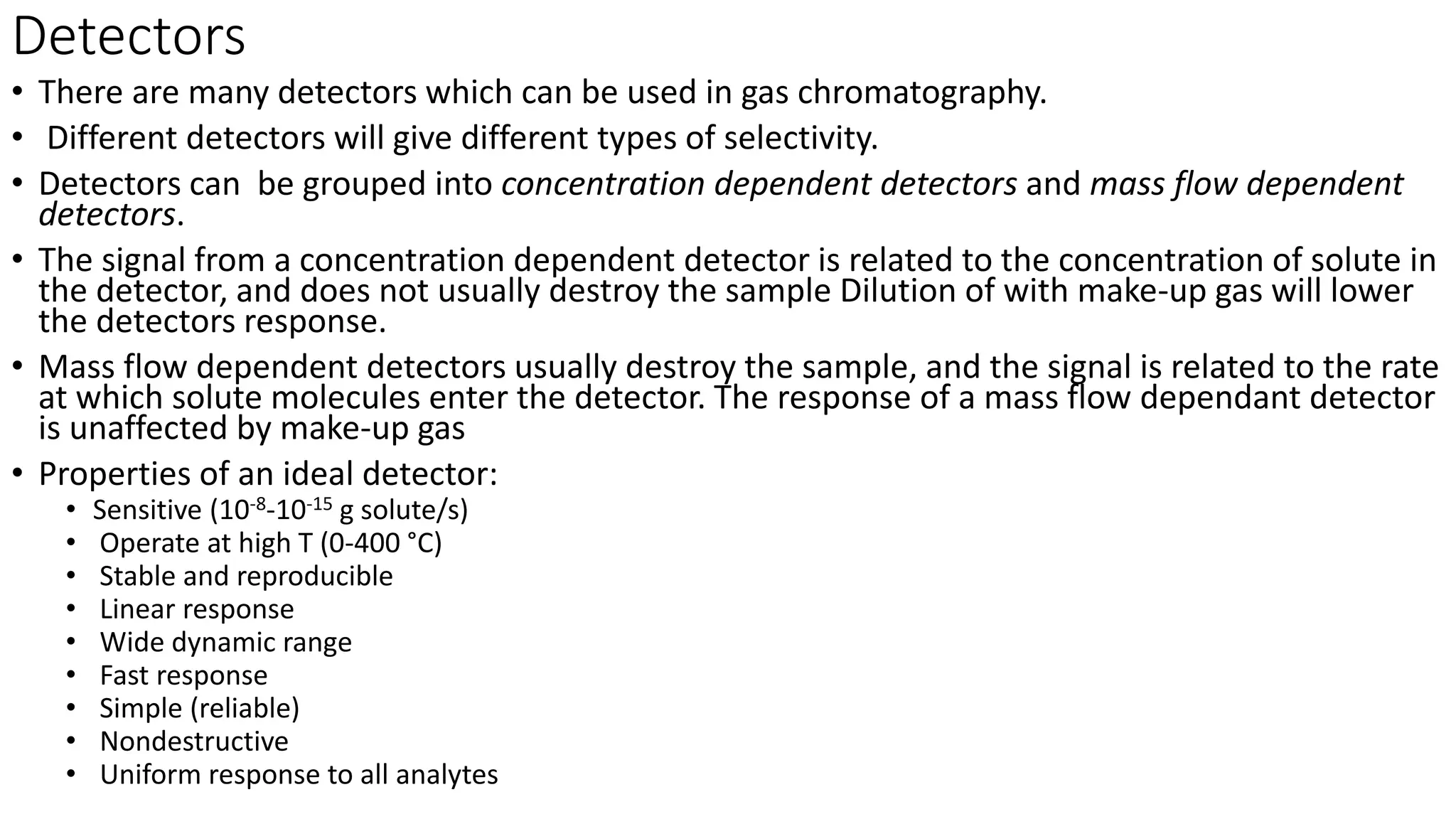
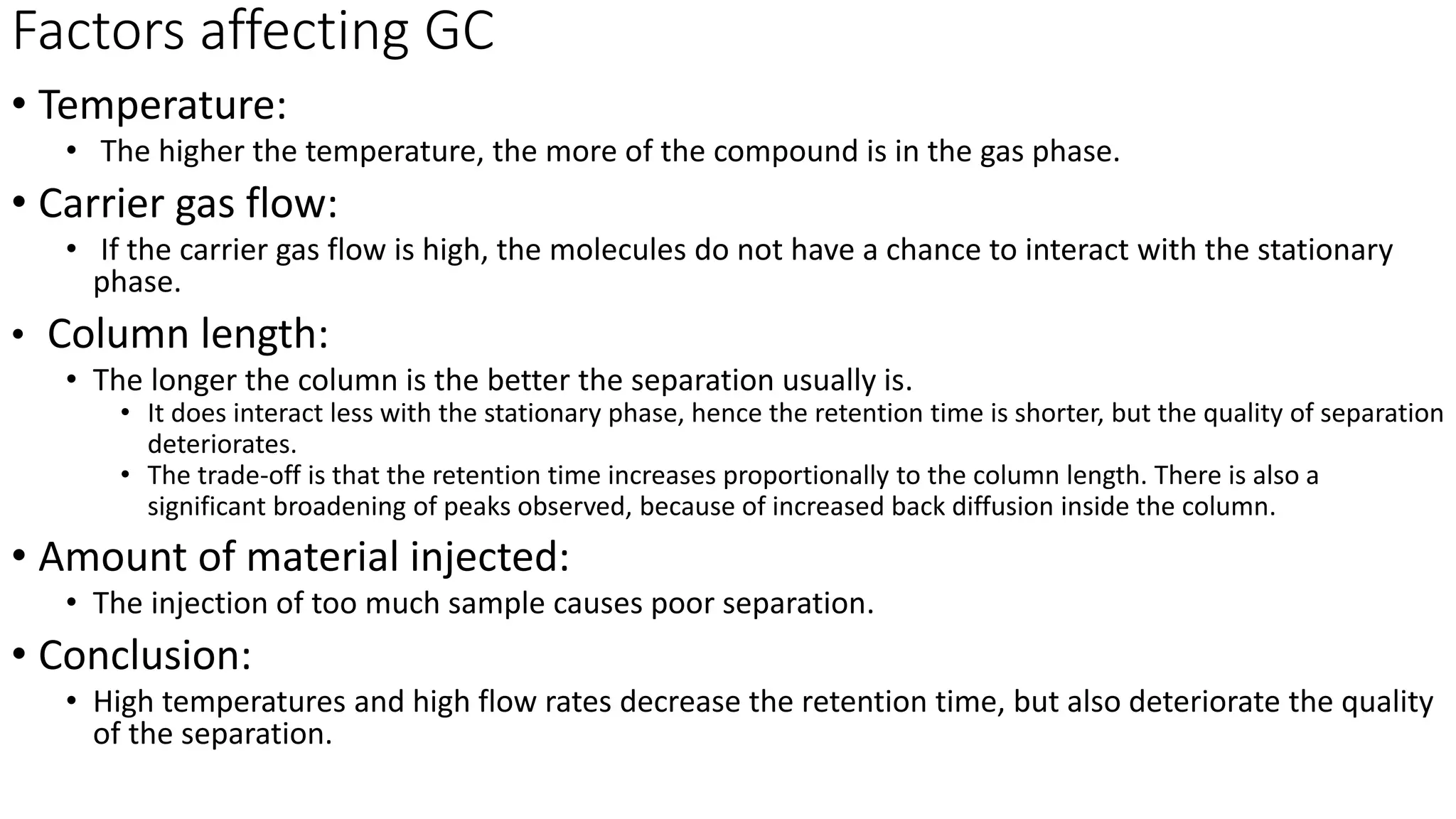
Chromatography techniques such as high performance liquid chromatography (HPLC), fast protein liquid chromatography (FPLC), and gas chromatography (GC) can be used to separate mixtures. HPLC uses high pressure to push a mobile phase through a column containing a stationary phase to separate complex mixtures. FPLC is a modified HPLC system designed for separating proteins more gently. GC vaporizes samples and uses an inert gas mobile phase to separate components in a sample based on differences in how they partition between the gas and a liquid or solid stationary phase.