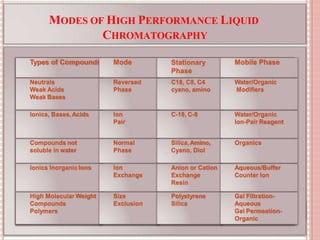
This document discusses high performance liquid chromatography (HPLC). It begins by defining chromatography and describing the basic principles of HPLC. It then discusses the types of HPLC separations based on modes, principles, elution techniques, scale of operation, and type of analysis. The document also covers the principles, types, and advantages of liquid chromatography. It provides details on the instrumentation of HPLC including solvent reservoirs, degassing, pumps, injectors, columns, detectors, and data handling. In summary:
HPLC is a type of column chromatography used to separate mixtures by distributing components between a stationary and mobile phase. It can be used for qualitative and quantitative analysis. The document outlines the various components of an HPLC system
![HIGH PERFORMANCE LIQUID
CHROMATOGRAPHY [HPLC]
Shinde Ganesh Shashikant
Assistant Professor
Pharmaceutical Analysis Dept.
Pravara College of Pharmacy ,Pravaranagar
1](https://image.slidesharecdn.com/newhplc-190826122638/85/HPLC-1-320.jpg)






![PRINCIPLE
High Performance Liquid Chromatography [HPLC] is principle is based on
adsorption as well as partition chromatography is depending on the nature
of stationary phase, if stationary phase is solid principle is based on adsorption
chromatography and if stationary phase is liquid principle is based on partition
chromatography.
It is important for determination of volatile and non volatile compounds.
It is important for determination qualitative and quantitative analysis.
It is important for determination of Retention Time (the time is required , after
sample injection maximum angle peak reaches to detector)
8](https://image.slidesharecdn.com/newhplc-190826122638/85/HPLC-8-320.jpg)