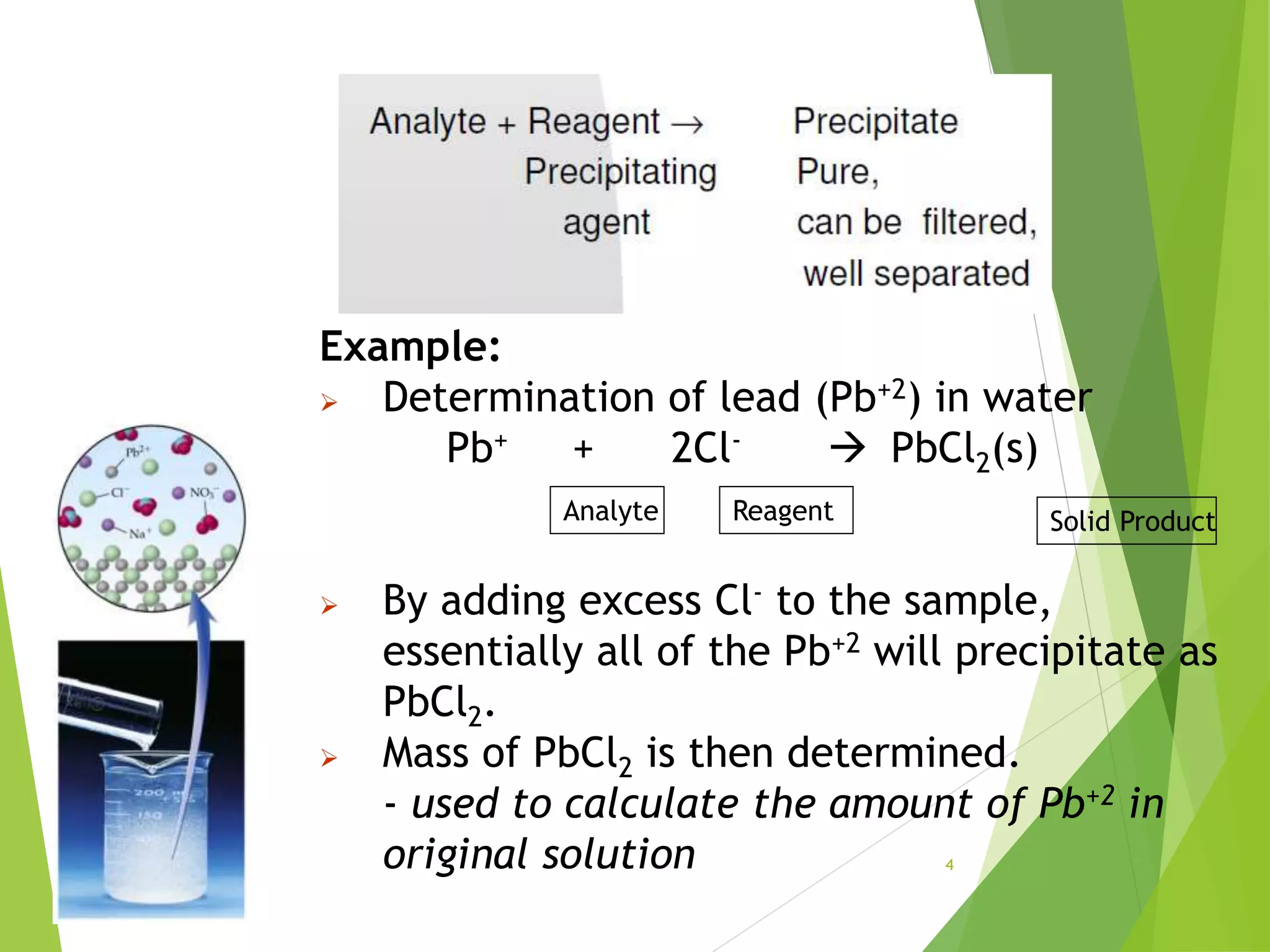
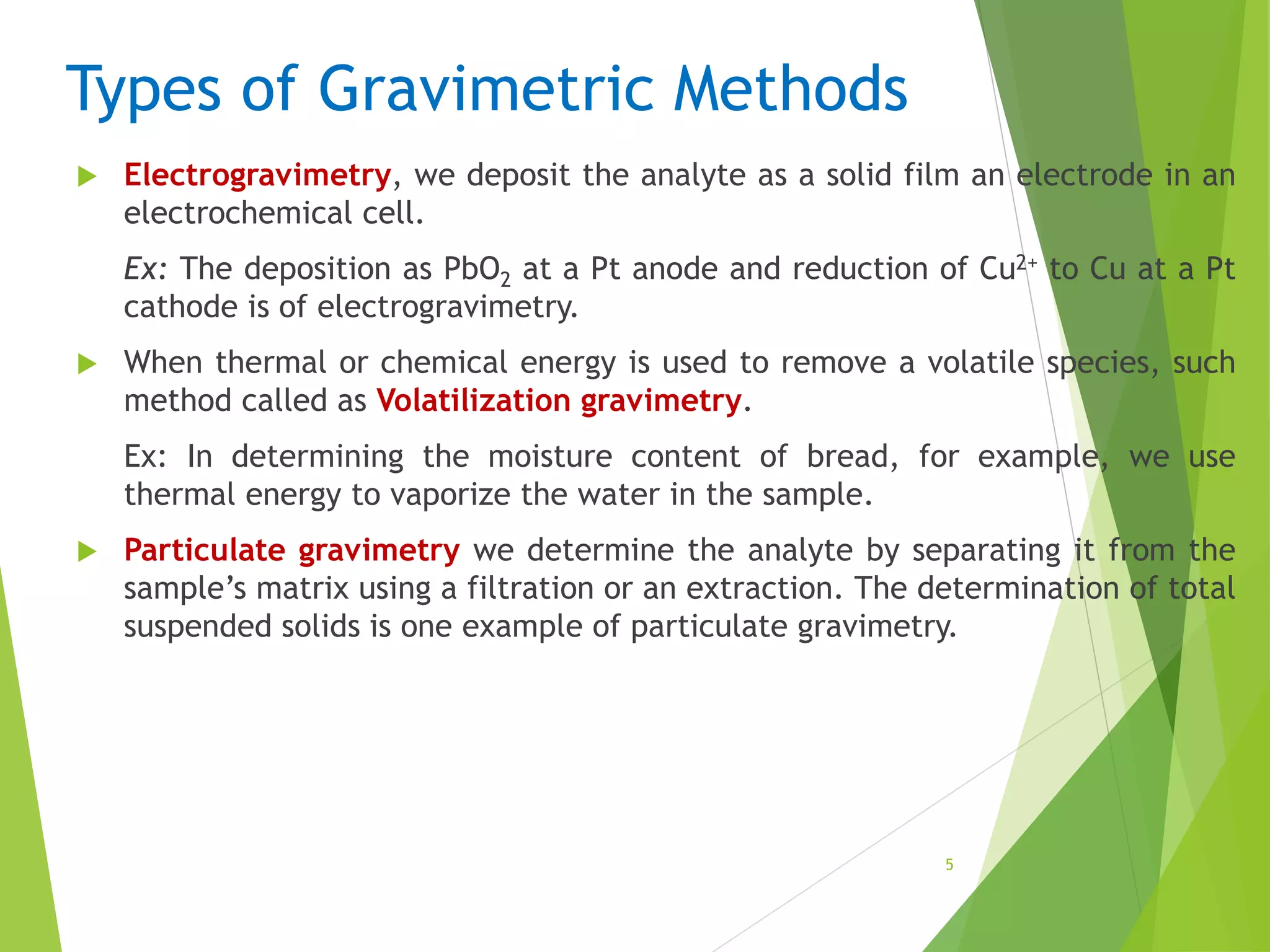
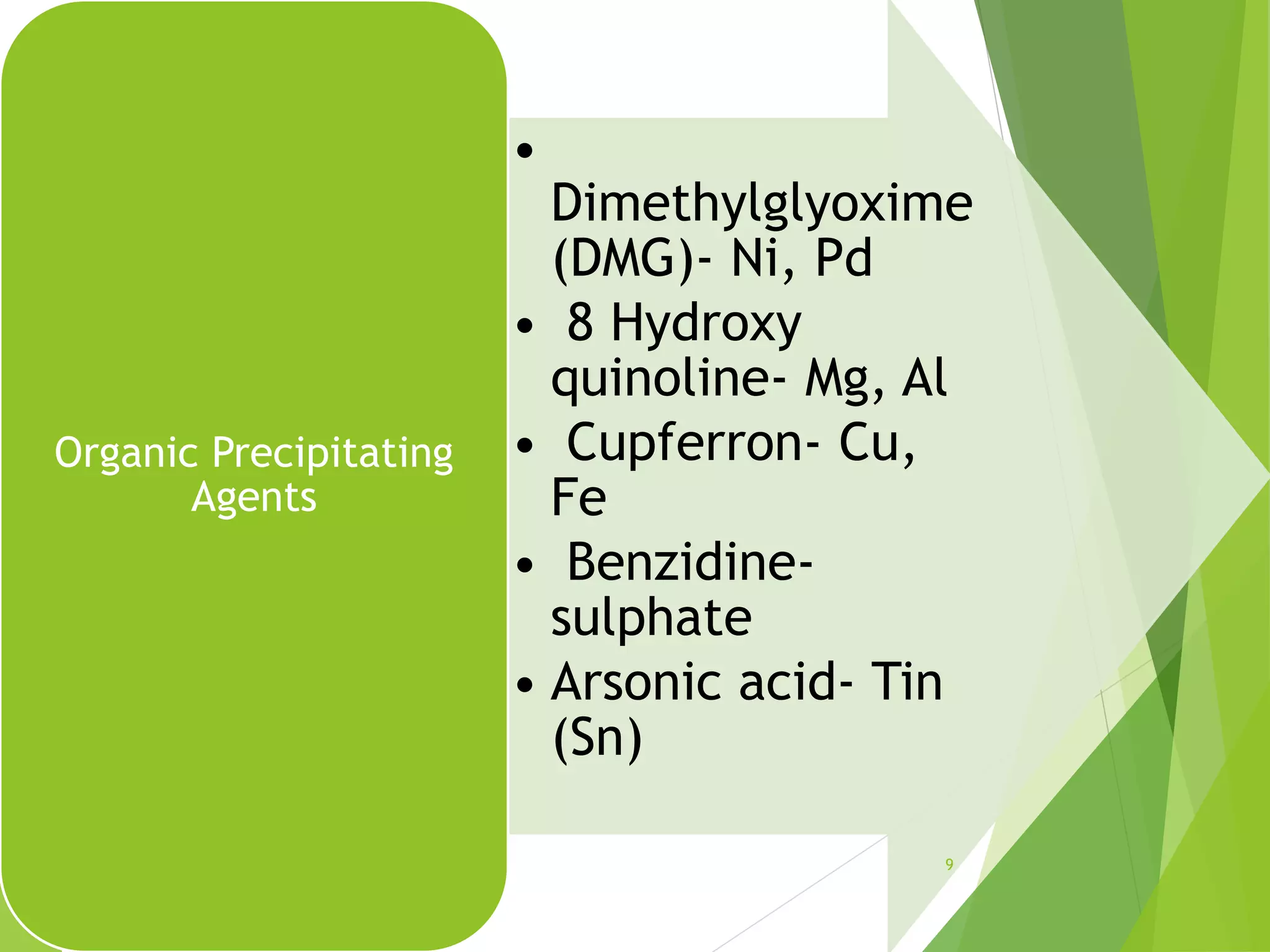
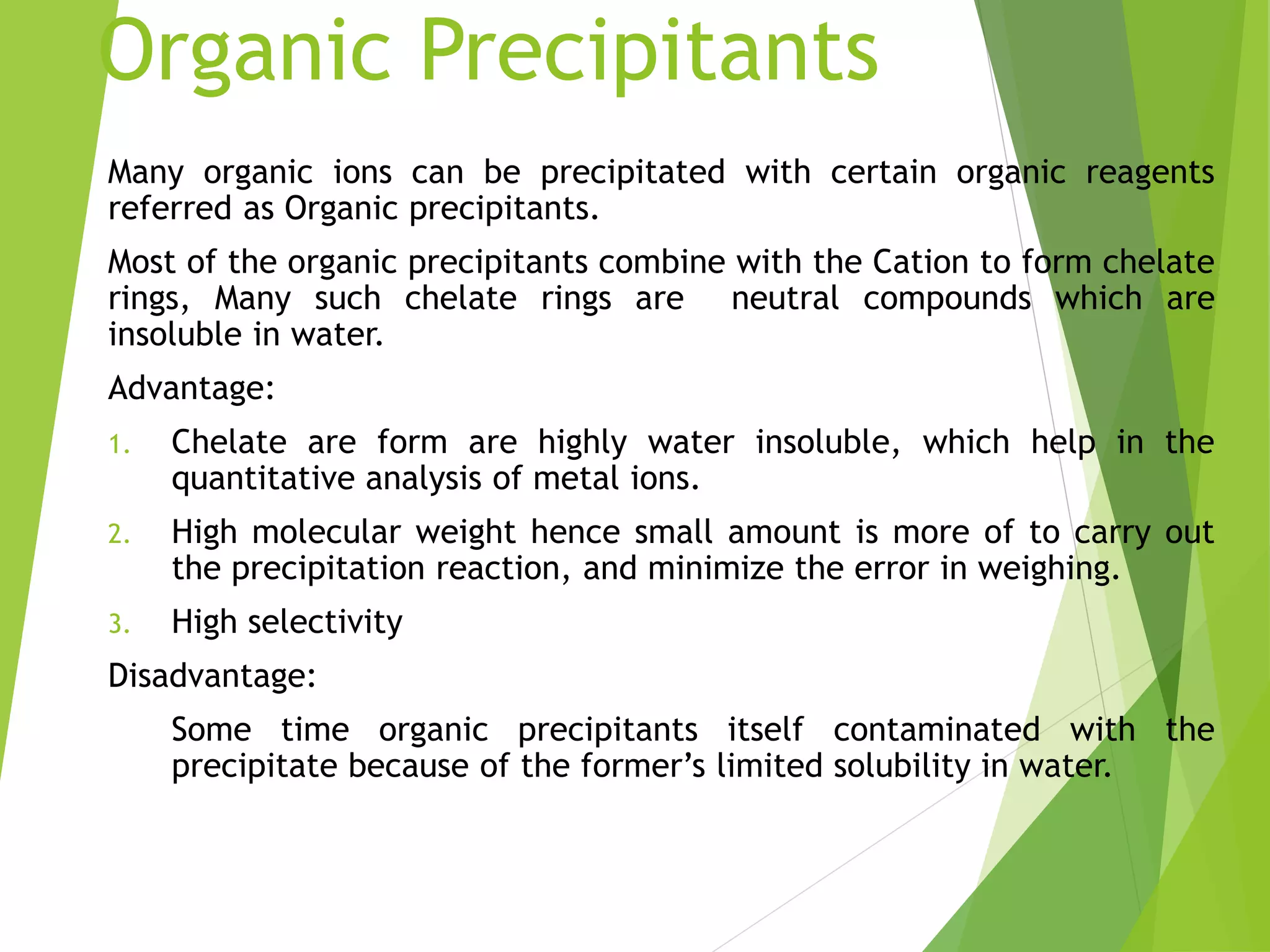
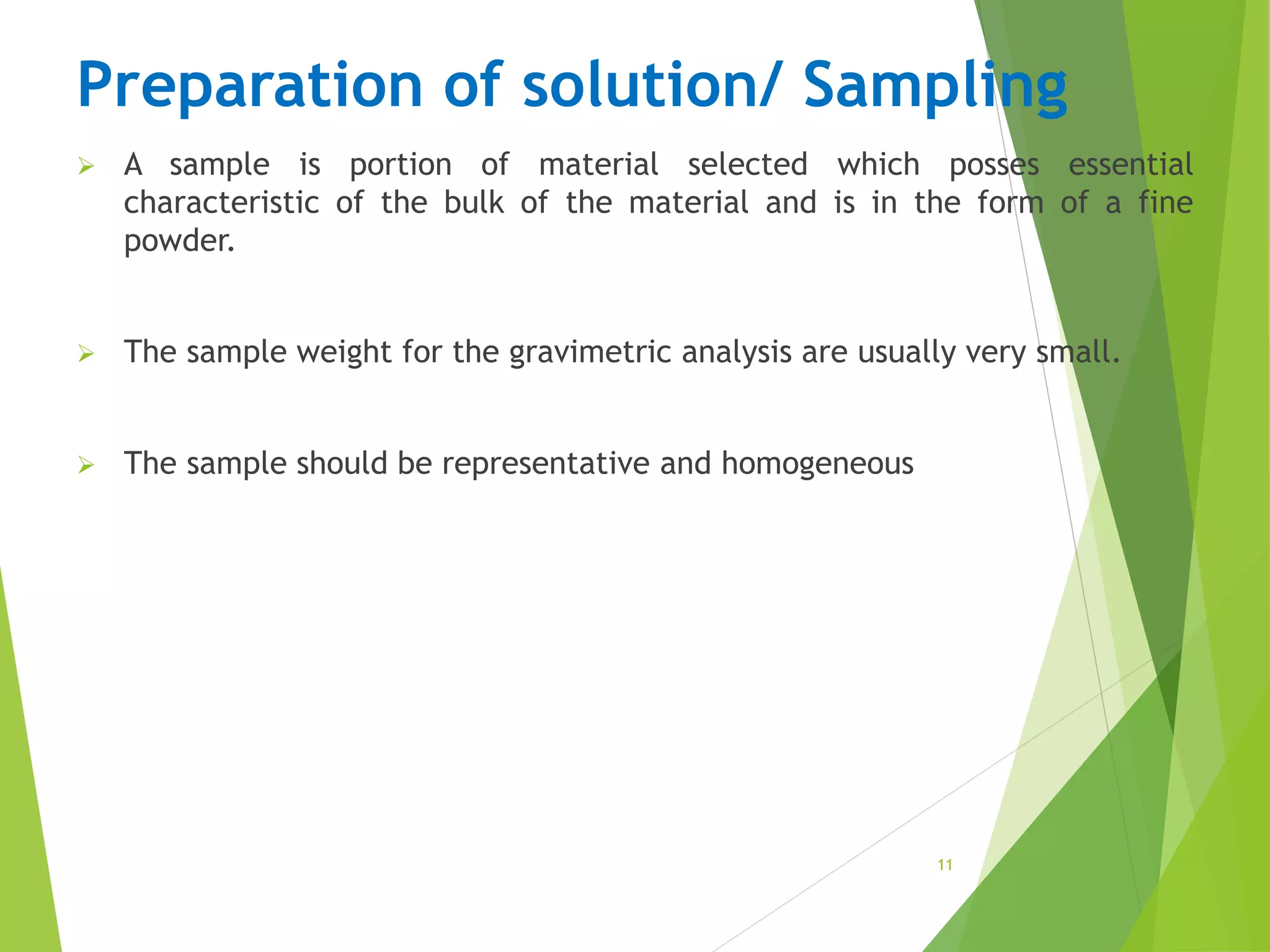
The document provides an overview of gravimetric analysis, defined as a quantitative method that isolates and weighs a specific element or compound. It outlines the types of gravimetric methods, including electrogravimetry, volatilization gravimetry, and particulate gravimetry, along with the key characteristics of precipitating agents and organic precipitants. Additionally, it emphasizes the importance of using representative and homogeneous samples in gravimetric analysis.