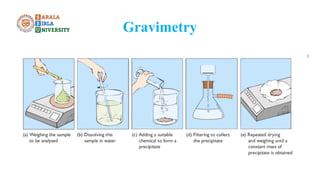
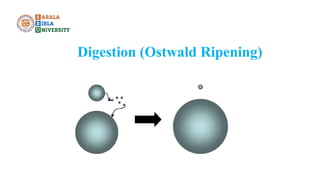
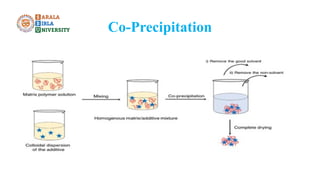
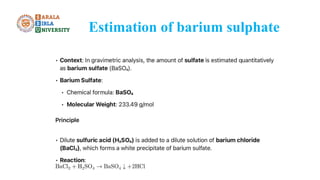
The document discusses gravimetric analysis, detailing its principles and essential steps including sampling, precipitation, digestion, filtration, washing, drying, weighing, and calculation. It emphasizes the importance of ensuring the purity of precipitates by managing co-precipitation and post-precipitation. Additionally, the document provides a practical example of estimating barium sulfate and outlines the applications of gravimetry in analyzing standard solutions.