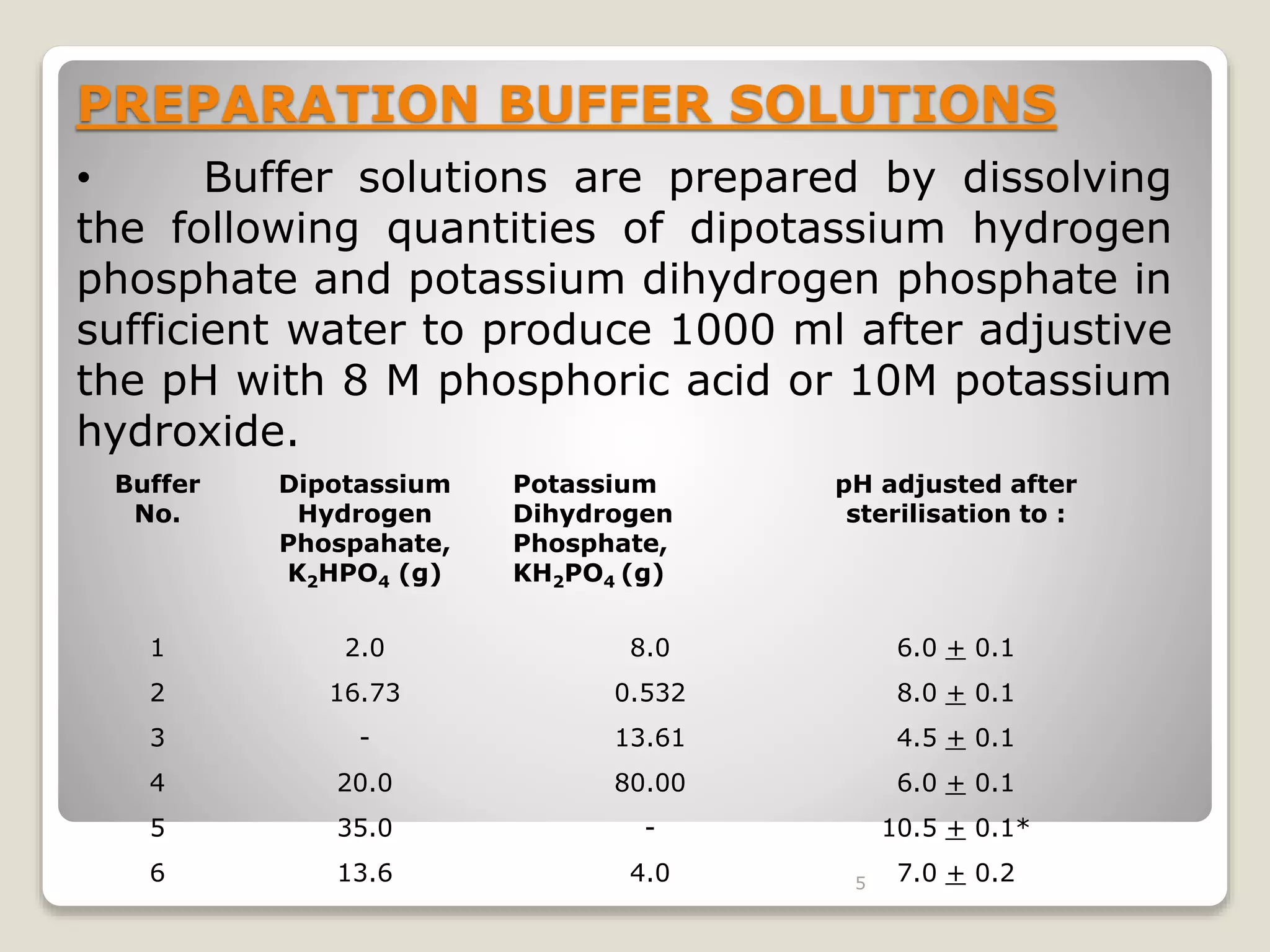
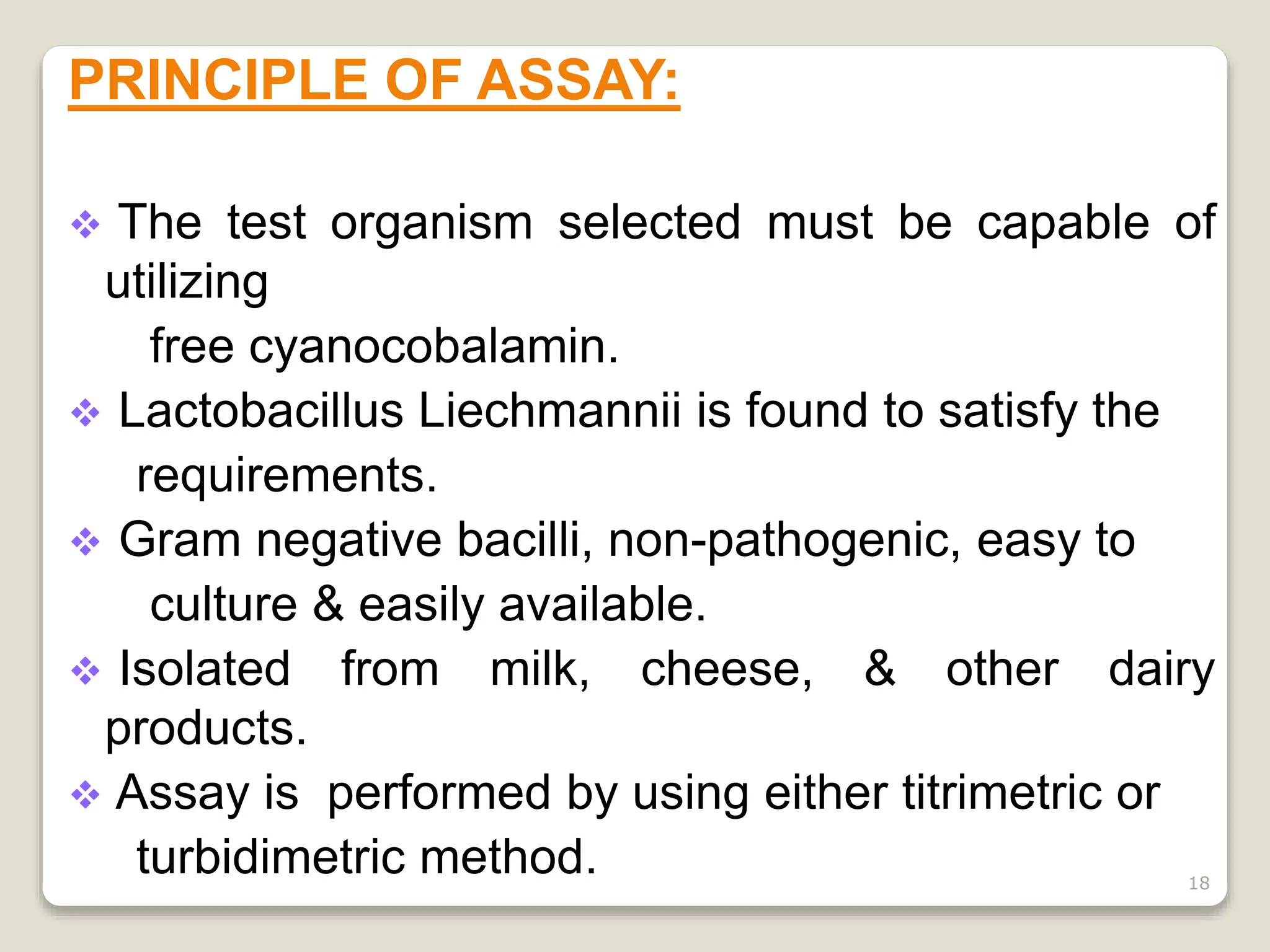
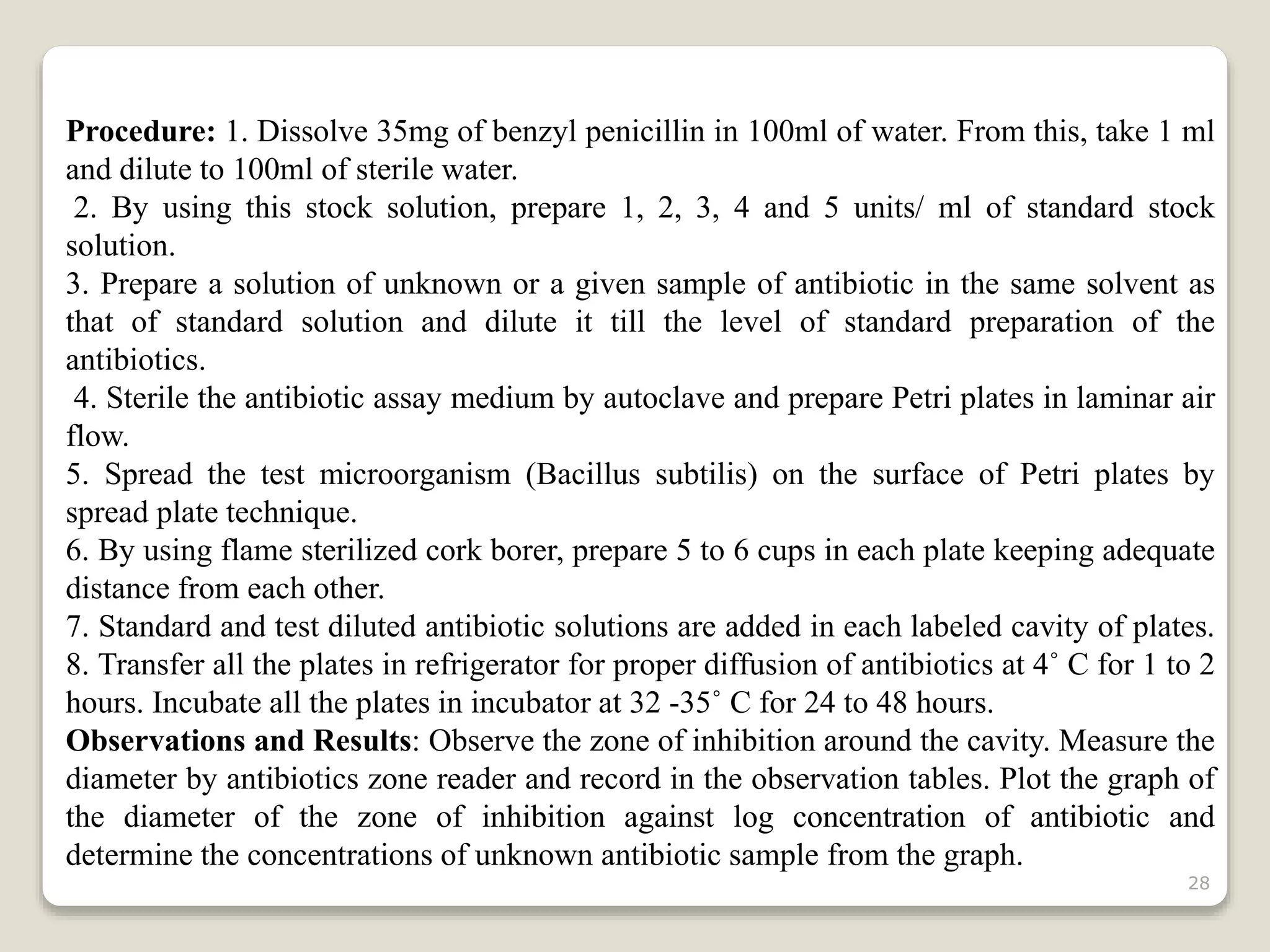
The document describes procedures for the microbiological assay of antibiotics and vitamins. There are two main methods for antibiotic assay - the cylinder-plate method and the turbidimetric method. The cylinder-plate method involves measuring zones of inhibition around cylinders containing different concentrations of antibiotics in an agar plate inoculated with a test microorganism. The turbidimetric method involves measuring inhibition of microbial growth in liquid medium containing varying antibiotic concentrations. Standards curves are constructed to determine unknown concentrations. Vitamin assays similarly rely on microbial growth responses to different concentrations of vitamins in culture media. Procedures for assaying vitamins B12 and B2 using specific test microorganisms like Lactobacillus leichmannii are provided.