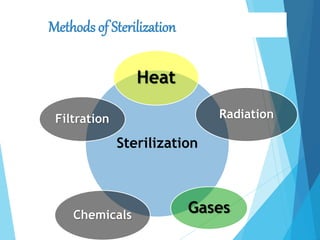
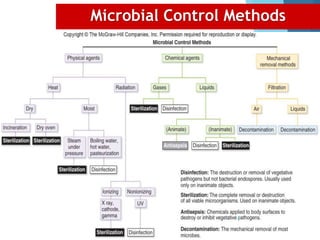
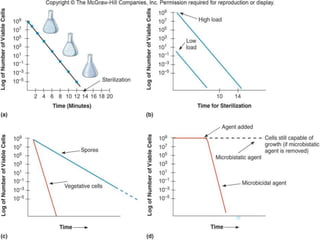
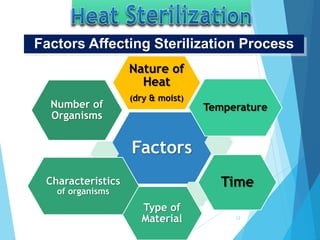
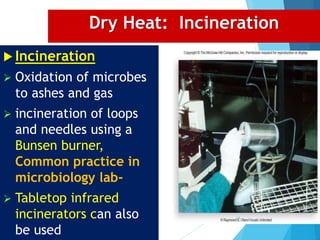
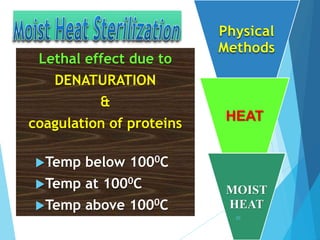
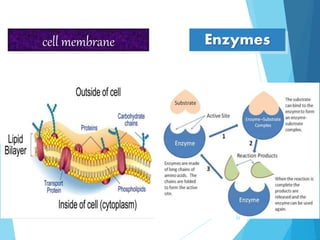
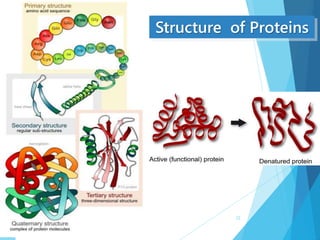
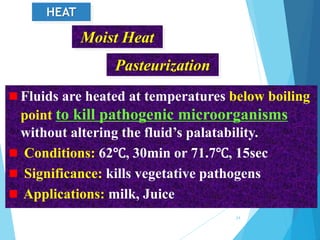
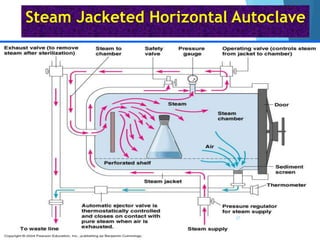
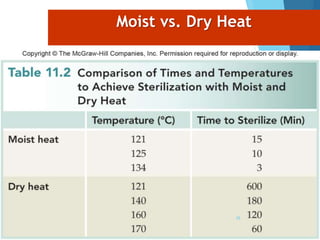
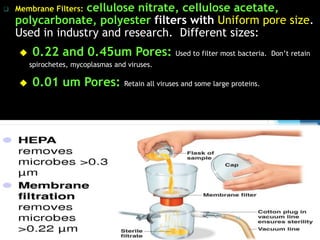
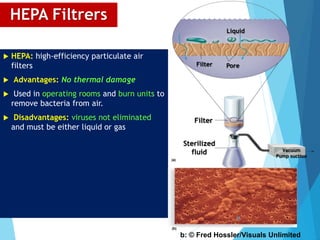
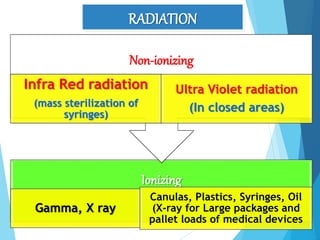
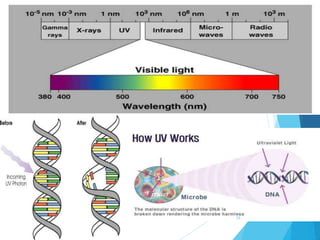
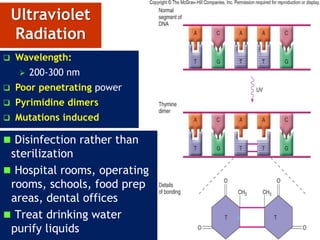
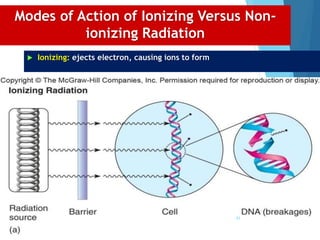
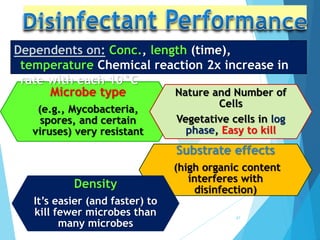
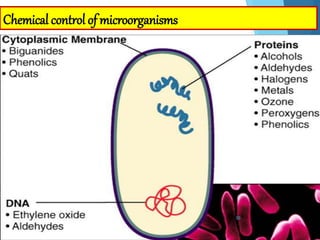
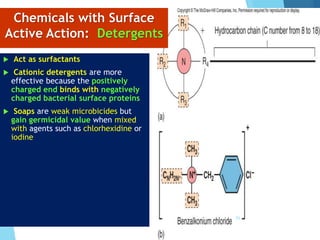
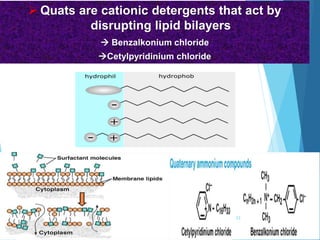
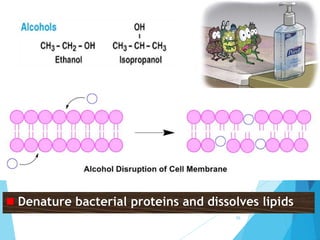
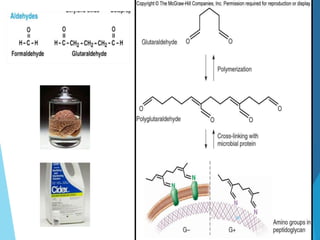
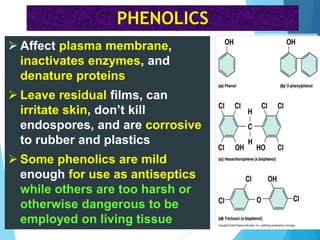
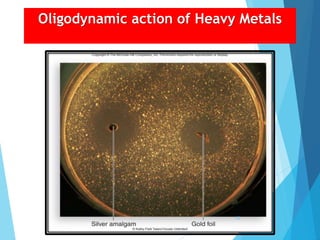
The document discusses various methods of sterilization and disinfection, focusing on their mechanisms, applications, and effectiveness against microorganisms. It outlines techniques such as heat, radiation, filtration, and chemical agents, while also defining terms like sterilization, disinfection, and antisepsis. Additionally, the document highlights the importance of monitoring sterilization processes and the characteristics of different disinfectants.