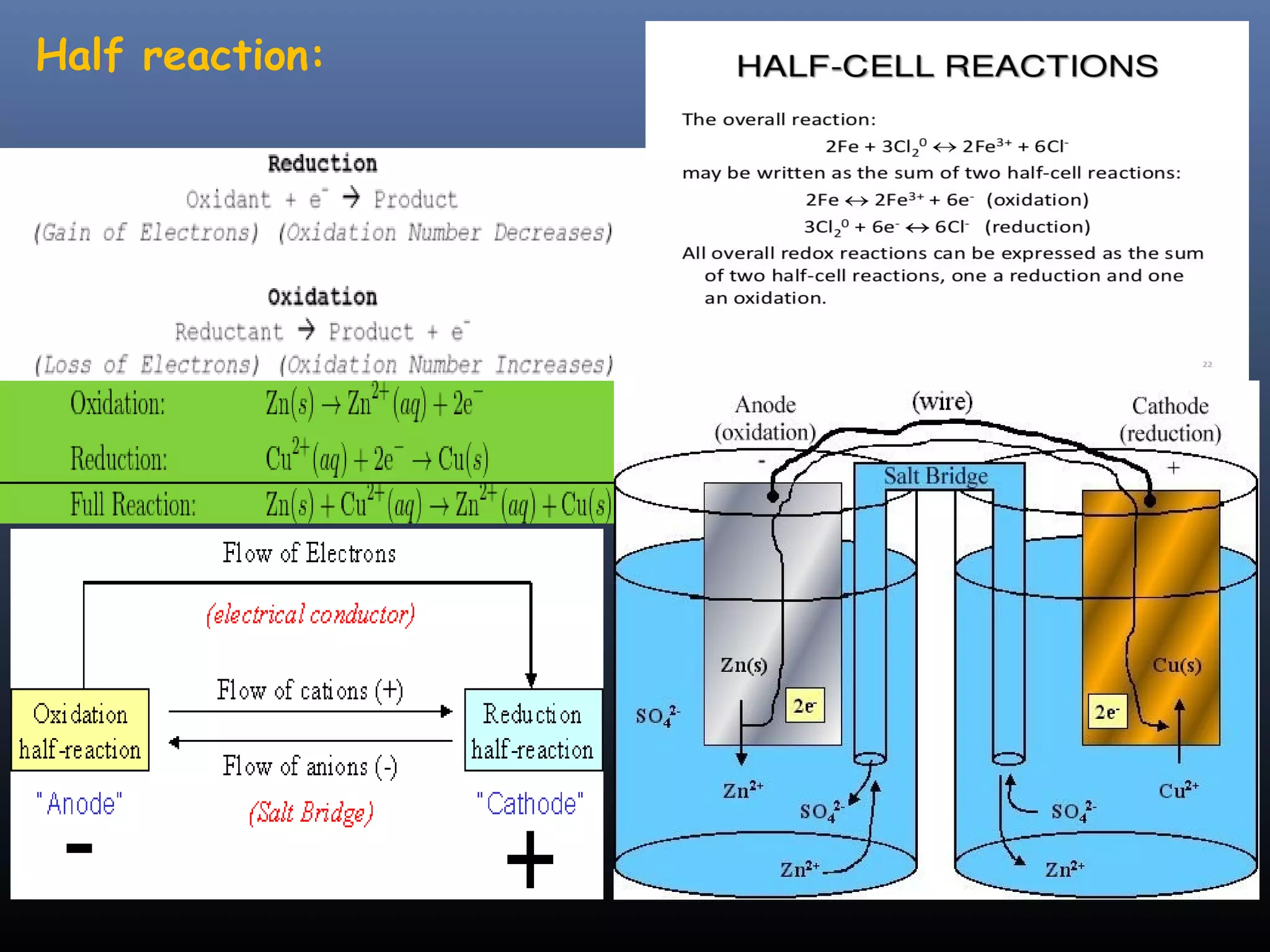
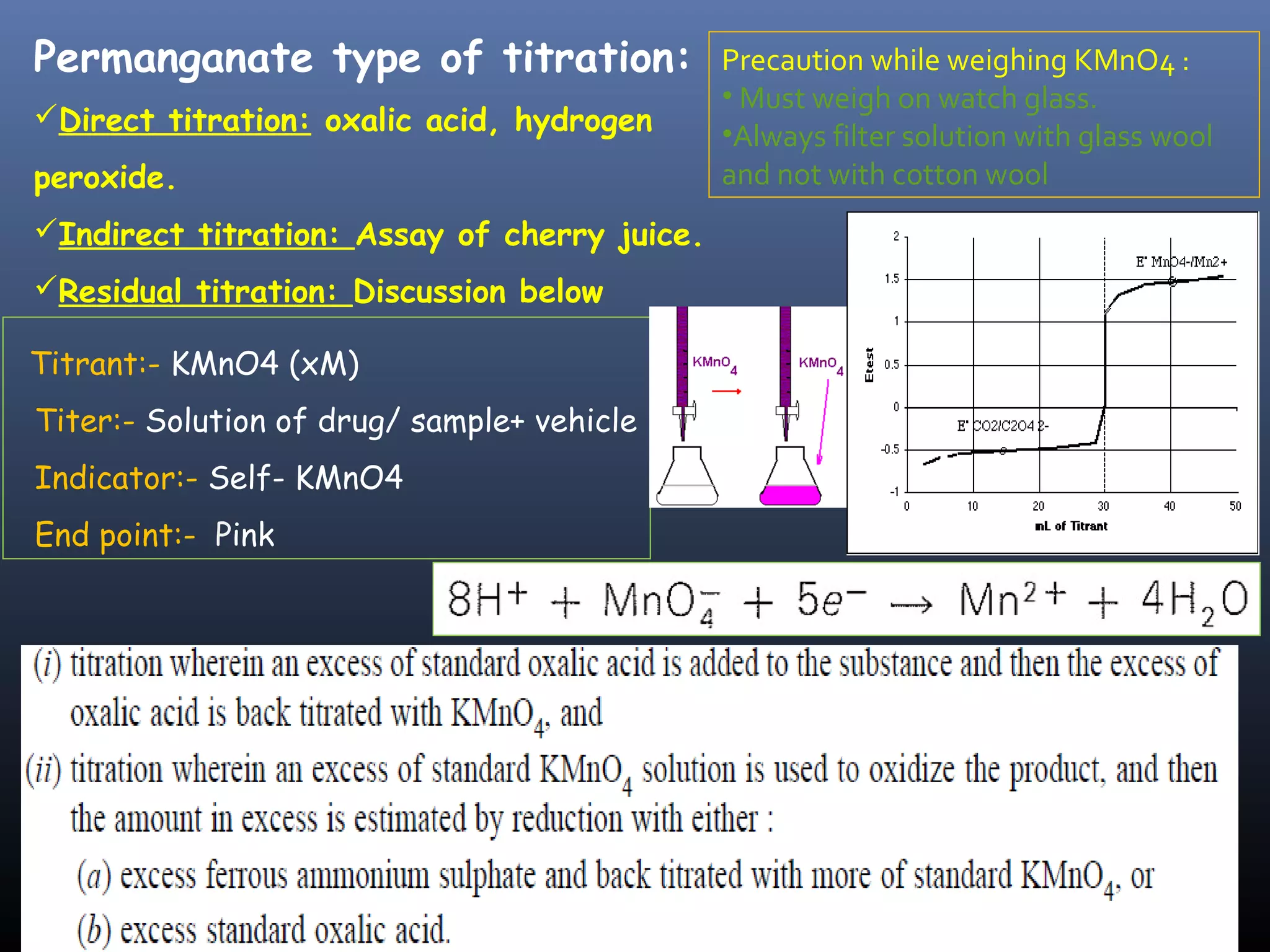
This document discusses various redox titration methods including permanganate, cerium, iodine, periodic acid, and sodium nitrite titrations. It provides details on relevant concepts such as redox equivalent weight calculations, types of indicators, and applications of different titration methods. Permanganate titrations can be direct, indirect, or residual and use potassium permanganate as the titrant. Cerium titrations use ammonium ceric sulfate and are advantageous over other methods. Sodium nitrite titration is used to determine primary aromatic amines. Periodic acid titration is used to determine polyhydroxy compounds by oxidation. Iodine titrations can be iodimetric or iod