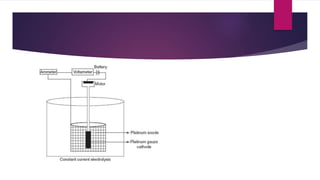
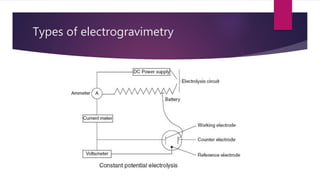
This document provides information about various gravimetric analysis techniques including precipitation gravimetry, volatilization gravimetry, electrogravimetry, and thermogravimetry. It explains that gravimetric analysis involves determining the mass of a compound or element through a chemical reaction such as precipitation or volatilization. Key steps like precipitation, filtration, drying and calculation are outlined for the common example of chloride analysis via silver chloride precipitation. Advantages and disadvantages of gravimetric analysis are also summarized.







































![Common ion effect
The solubility of a salt is less in a solution containing ions in common with that of the
salt in water provided the equilibrium is not disturbed by the formation of complex
ions this is known as common ion effect.
The solubility of precipitate is decreased by the presence of other ions in the solution
common with the precipitate. E.g. during the precipitation of Ag+ ions from an
aqueous solution containing excess of Cl- ions, even small quantity of Ag+ ions in the
solution can get readily precipitated as Cl-ions are present in excess. product, Ksp of
AgCl is constant, Ksp = [Ag+] [Cl-]In some cases excess of precipitant decreases the
solubility of precipitate but in some cases it increases.
In some gravimetric estimation, organic reagents are used as precipitant which are
prepared in non-polar organic solvents. E.g. dimethylglyoximate (DMG) for
precipitation of nckel present in solution.](https://image.slidesharecdn.com/gravimetry-220919150539-aa270439/85/Gravimetry-pptx-42-320.jpg)
![Uncommon or Diverse Ion Effect
Solubility of a sparingly soluble salt increases in presence of foreign ion, that is
ions which are not common to those of the salt. E.g. Solubility of BaSO4 is
increased by 70% in 0.01M solution of potassium nitrate than in water. Potassium
nitrate is strong electrolyte and highly soluble in water and its solution contains a
high conc. of ions.
This results increase of ionic strength of the solution.
The activity ‘a’ is related to the molar concentration ‘c’ by the activity coefficient ‘γ’
as a = c * γ
e.g. solubility product Ksp for AgCl is written as Ksp = a Ag+ * a Cl- = [cAg+ * γ
Ag+] x [cCl- * γ Cl-]](https://image.slidesharecdn.com/gravimetry-220919150539-aa270439/85/Gravimetry-pptx-43-320.jpg)

