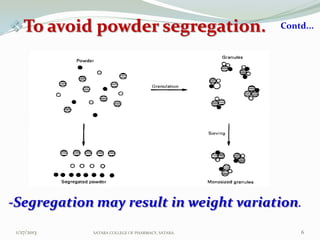
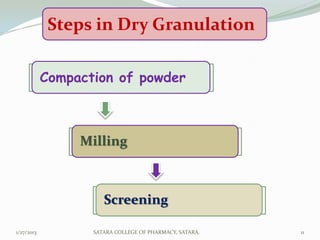
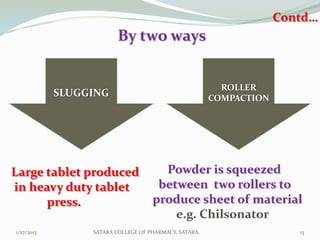
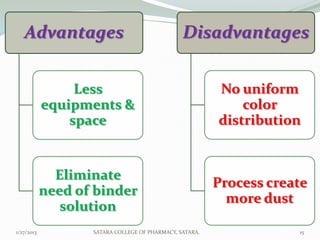
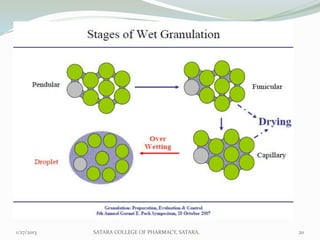
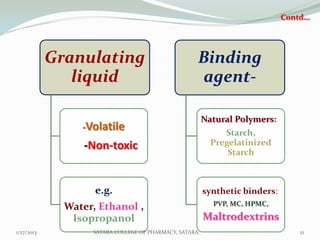
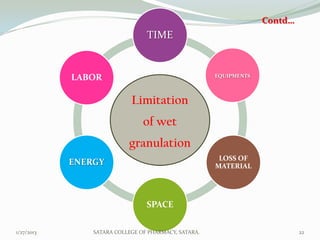
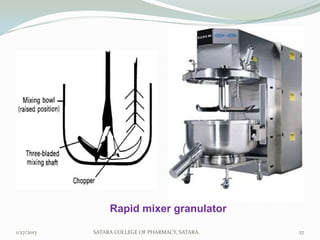
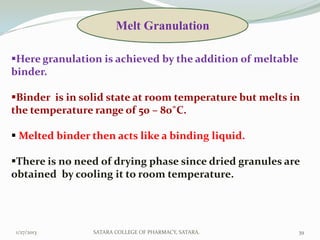
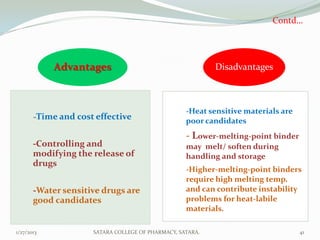
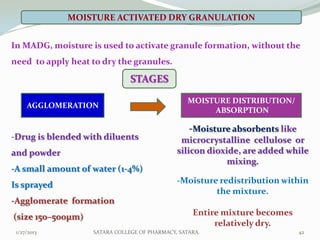
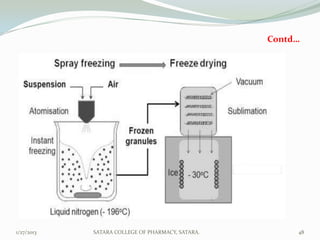
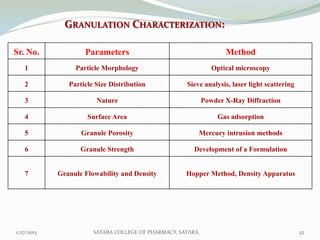
The document discusses various granulation techniques used in pharmaceutical manufacturing. It begins with an introduction to granules and granulation. It then covers different granulation methods including dry granulation, wet granulation and advanced techniques like fluid bed granulation, extrusion-spheronization, steam granulation and melt granulation. The document provides details on the process, equipment used, advantages and disadvantages of each method. It aims to explain why granulation is important and the various ways it can be achieved.