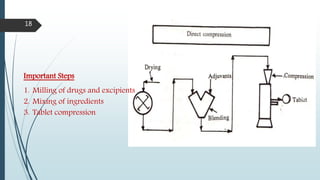
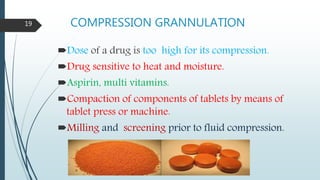
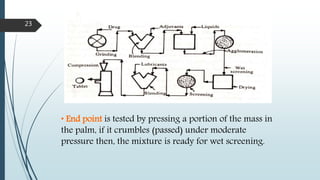
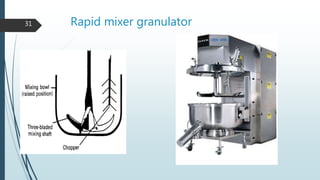
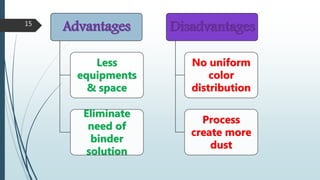
This document provides information about tablets, including their formulation, design, manufacturing, types, advantages, and excipients. Tablets are solid oral dosage forms made by compressing powder mixtures into various shapes. They have advantages like precise dosing and ease of production. The document discusses different granulation and compression methods used in tablet manufacturing. It also describes common excipients like diluents, binders, disintegrants, lubricants and their functions in tablets.











![Drug
Excipients
Diluents
Disintegrant
Large sized tablets
Slugs
Granules
Disintegrant
Glidant
Lubricant
tablets
Compression
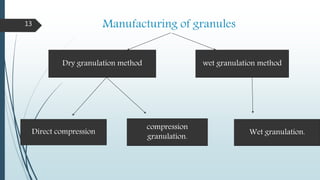
granulation
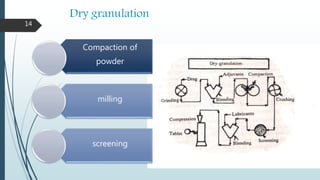
17
Weighing
Mixing [blender]
Slugging[tablet press,
roller compactor.
Screening sieve[20-25]
Mixing
Compression [tablet
press]](https://image.slidesharecdn.com/tabletsformulation-170202061350/85/Tablets-formulation-17-320.jpg)