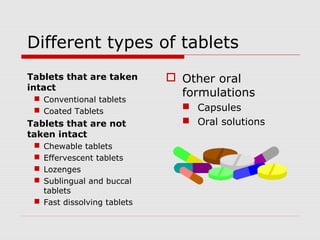
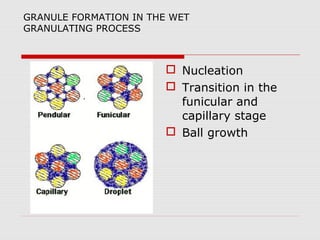
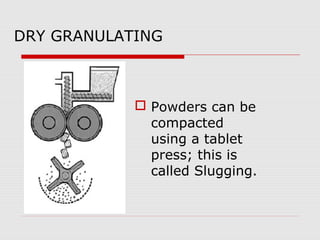
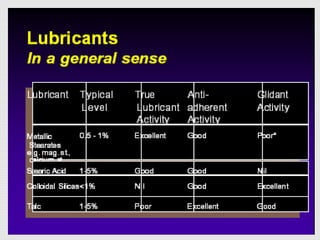
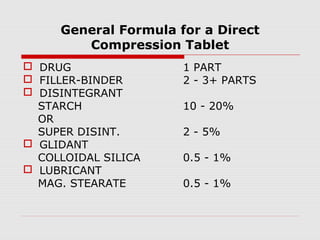
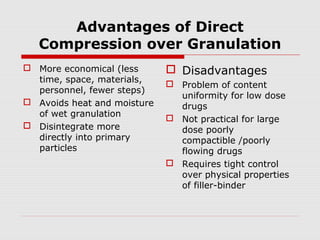
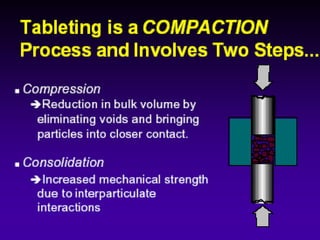
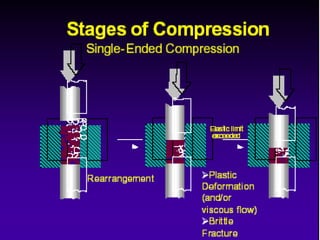
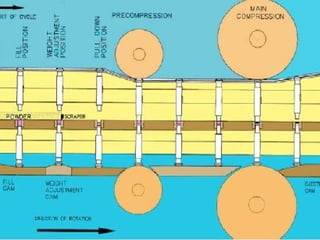
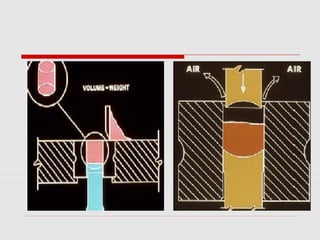
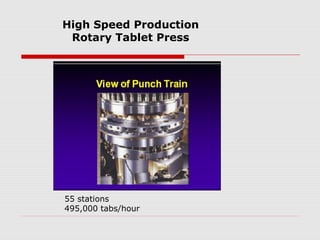
Tablets are one of the most common oral drug delivery forms, comprising around half of all pharmaceutical products. Tablets offer advantages like high patient compliance and ease of production and marketing. However, some substances may not be well absorbed in the gastrointestinal tract. Tablets are compressed dispersions of particles that come in various types depending on if they are taken intact or not. Their manufacture involves steps like raw material procurement, formulation, granulation, compression, coating and packaging. Granulation can improve properties like flow and compressibility and is usually done by wet or dry processes. Tablets contain active substances and excipients like fillers, disintegrants, binders, lubricants and coatings to provide the desired properties.