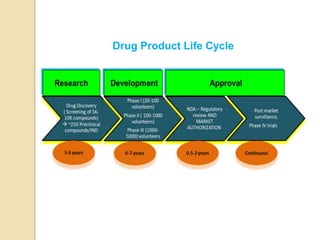
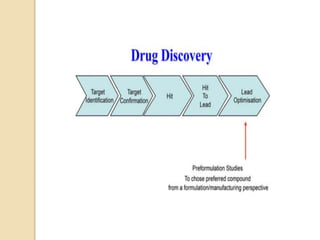
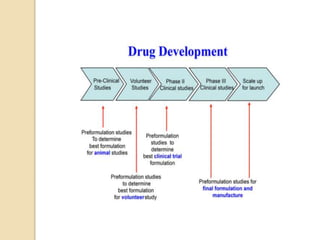
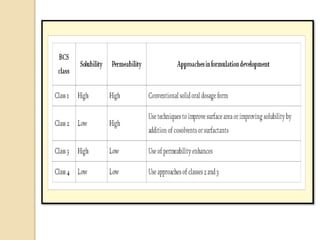
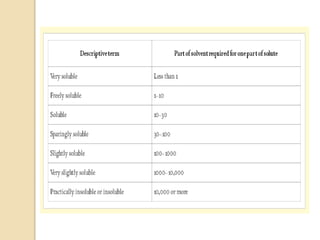
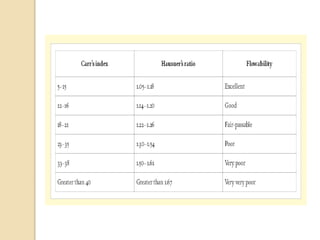
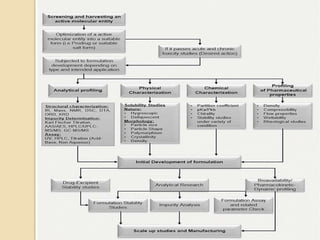
This document discusses preformulation studies, which characterize a drug molecule's physical and chemical properties to develop a safe, effective, and stable dosage form. Preformulation helps assess a molecule's suitability for development and reduces risks. It provides data to screen lead compounds and guide appropriate dosage design. The objective is to select the right drug substance, excipients, and packaging to minimize issues in later development stages. Key preformulation study parameters include solubility, permeability, compatibility, and properties like pH and polymorphism. Preformulation assists both drug discovery and development.